Abstract
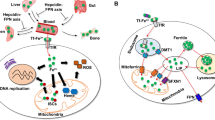
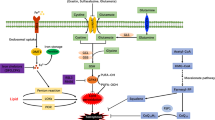
The transition metal iron is essential for life, yet potentially toxic iron-catalyzed reactive oxygen species (ROS) are unavoidable in an oxygen-rich environment. Iron and ROS are increasingly recognized as important initiators and mediators of cell death in a variety of organisms and pathological situations. Here, we review recent discoveries regarding the mechanism by which iron and ROS participate in cell death. We describe the different roles of iron in triggering cell death, targets of iron-dependent ROS that mediate cell death and a new form of iron-dependent cell death termed ferroptosis. Recent advances in understanding the role of iron and ROS in cell death offer unexpected surprises and suggest new therapeutic avenues to treat cancer, organ damage and degenerative disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Frey, P.A. & Reed, G.H. The ubiquity of iron. ACS Chem. Biol. 7, 1477–1481 (2012).
Lambeth, J.D. & Neish, A.S. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. doi:10.1146/annurev-pathol-012513-104651 (2013).
Kell, D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genomics 2, 2 (2009).
Petrat, F., de Groot, H., Sustmann, R. & Rauen, U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383, 489–502 (2002).
Kurz, T., Eaton, J.W. & Brunk, U.T. The role of lysosomes in iron metabolism and recycling. Int. J. Biochem. Cell Biol. 43, 1686–1697 (2011).
Carlioz, A. & Touati, D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5, 623–630 (1986).
Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 (2013).
Flint, D.H., Tuminello, J.F. & Emptage, M.H. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268, 22369–22376 (1993).
Esposito, L.A., Melov, S., Panov, A., Cottrell, B.A. & Wallace, D.C. Mitochondrial disease in mouse results in increased oxidative stress. Proc. Natl. Acad. Sci. USA 96, 4820–4825 (1999).
Melov, S. et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 96, 846–851 (1999).
Li, Y. et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11, 376–381 (1995).
Brennan, A.M. et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 12, 857–863 (2009).
Farrow, M.A. et al. Clostridium difficile toxin B–induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc. Natl. Acad. Sci. USA 110, 18674–18679 (2013).
Seaver, L.C. & Imlay, J.A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183, 7173–7181 (2001).
Park, S., You, X. & Imlay, J.A. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx− mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 102, 9317–9322 (2005).
Jang, S. & Imlay, J.A. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282, 929–937 (2007).
Jang, S. & Imlay, J.A. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol. Microbiol. 78, 1448–1467 (2010).
Anjem, A. & Imlay, J.A. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 287, 15544–15556 (2012).
Sobota, J.M. & Imlay, J.A. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. USA 108, 5402–5407 (2011).
Cvetkovic, A. et al. Microbial metalloproteomes are largely uncharacterized. Nature 466, 779–782 (2010).
Antunes, F., Cadenas, E. & Brunk, U.T. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem. J. 356, 549–555 (2001).
Hamacher-Brady, A. et al. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 286, 6587–6601 (2011).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Heo, J.M. et al. A stress-responsive system for mitochondrial protein degradation. Mol. Cell 40, 465–480 (2010).
Madeo, F., Frohlich, E. & Frohlich, K.U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139, 729–734 (1997).
Braun, R.J. et al. Crucial mitochondrial impairment upon CDC48 mutation in apoptotic yeast. J. Biol. Chem. 281, 25757–25767 (2006).
Madeo, F. et al. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767 (1999).
Bartolome, F. et al. Pathogenic VCP mutations induce mitochondrial uncoupling and reduced ATP levels. Neuron 78, 57–64 (2013).
Raimundo, N. et al. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell 148, 716–726 (2012).
Chae, S. et al. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal. 6, rs4 (2013).
Kagan, V.E. et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 (2005).
Ji, J. et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1413 (2012).
Jiang, J. et al. Interplay between bax, reactive oxygen species production, and cardiolipin oxidation during apoptosis. Biochem. Biophys. Res. Commun. 368, 145–150 (2008).
Ricci, J.E. et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117, 773–786 (2004).
Huai, J. et al. TNFa-induced lysosomal membrane permeability (LMP) is downstream of MOMP and triggered by caspase-mediated p75 cleavage and ROS formation. J. Cell Sci. 126, 4015–4025 (2013).
Garcia-Perez, C. et al. Bid-induced mitochondrial membrane permeabilization waves propagated by local reactive oxygen species (ROS) signaling. Proc. Natl. Acad. Sci. USA 109, 4497–4502 (2012).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 110, 12024–12029 (2013).
Zhang, D.W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Shulga, N. & Pastorino, J.G. GRIM-19–mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J. Cell Sci. 125, 2995–3003 (2012).
Kim, Y.S., Morgan, M.J., Choksi, S. & Liu, Z.G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687 (2007).
Yazdanpanah, B. et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460, 1159–1163 (2009).
Xie, C. et al. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor–induced death in L929 cells. Mol. Cell. Biol. 25, 6673–6681 (2005).
Dixon, S.J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yagoda, N. et al. RAS-RAF-MEK–dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007).
Yang, W.S. & Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
Gout, P.W., Buckley, A.R., Simms, C.R. & Bruchovsky, N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 (2001).
Murphy, T.H., Miyamoto, M., Sastre, A., Schnaar, R.L. & Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2, 1547–1558 (1989).
Wolpaw, A.J. et al. Modulatory profiling identifies mechanisms of small molecule–induced cell death. Proc. Natl. Acad. Sci. USA 108, E771–E780 (2011).
Shaw, A.T. et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl. Acad. Sci. USA 108, 8773–8778 (2011).
Siddiq, A. et al. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J. Neurosci. 29, 8828–8838 (2009).
Li, Y., Maher, P. & Schubert, D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19, 453–463 (1997).
Abeysinghe, R.D. et al. The environment of the lipoxygenase iron binding site explored with novel hydroxypyridinone iron chelators. J. Biol. Chem. 271, 7965–7972 (1996).
Tan, S., Schubert, D. & Maher, P. Oxytosis: A novel form of programmed cell death. Curr. Top. Med. Chem. 1, 497–506 (2001).
Zaman, K. et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. 19, 9821–9830 (1999).
Tan, S., Wood, M. & Maher, P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J. Neurochem. 71, 95–105 (1998).
Tobaben, S. et al. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 18, 282–292 (2011).
Henke, N. et al. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 4, e470 (2013).
Volpe, J.J., Kinney, H.C., Jensen, F.E. & Rosenberg, P.A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440 (2011).
Sato, H. et al. Distribution of cystine/glutamate exchange transporter, system xc−, in the mouse brain. J. Neurosci. 22, 8028–8033 (2002).
Hentze, M.W., Muckenthaler, M.U., Galy, B. & Camaschella, C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010).
Puccio, H. et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27, 181–186 (2001).
Eaton, J.W. & Qian, M. Molecular bases of cellular iron toxicity. Free Radic. Biol. Med. 32, 833–840 (2002).
Silva-Gomes, S. et al. Transcription factor NRF2 protects mice against dietary iron–induced liver injury by preventing hepatocytic cell death. J. Hepatol. doi:10.1016/j.jhep.2013.09.004 (2013).
Torti, S.V. & Torti, F.M. Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13, 342–355 (2013).
Kruer, M.C. The neuropathology of neurodegeneration with brain iron accumulation. Int. Rev. Neurobiol. 110, 165–194 (2013).
Lei, P. et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 18, 291–295 (2012).
Duce, J.A. et al. Iron-export ferroxidase activity of b-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell 142, 857–867 (2010).
Kaur, D. et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37, 899–909 (2003).
Guzman, J.N. et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468, 696–700 (2010).
Allen, G.F., Toth, R., James, J. & Ganley, I.G. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. doi:10.1038/embor.2013.168 (2013).
Chen, Y. et al. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J. Neurosci. 33, 3582–3587 (2013).
Cheah, J.H. et al. NMDA receptor–nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51, 431–440 (2006).
Vaseva, A.V. et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 (2012).
Zhang, X. & Lemasters, J.J. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic. Biol. Med. 63, 243–253 (2013).
Uchiyama, A. et al. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress–induced hepatocellular injury. Hepatology 48, 1644–1654 (2008).
Li, L., Chen, O.S., McVey Ward, D. & Kaplan, J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276, 29515–29519 (2001).
Lin, H., Li, L., Jia, X., Ward, D.M. & Kaplan, J. Genetic and biochemical analysis of high iron toxicity in yeast: iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions. J. Biol. Chem. 286, 3851–3862 (2011).
Holmes-Hampton, G.P., Jhurry, N.D., McCormick, S.P. & Lindahl, P.A. Iron content of Saccharomyces cerevisiae cells grown under iron-deficient and iron-overload conditions. Biochemistry 52, 105–114 (2013).
Li, L., Bagley, D., Ward, D.M. & Kaplan, J. Yap5 is an iron-responsive transcriptional activator that regulates vacuolar iron storage in yeast. Mol. Cell. Biol. 28, 1326–1337 (2008).
Lee, Y.J. et al. Sphingolipid signaling mediates iron toxicity. Cell Metab. 16, 90–96 (2012).
Breslow, D.K. et al. Orm family proteins mediate sphingolipid homeostasis. Nature 463, 1048–1053 (2010).
Kelso, G.F. et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596 (2001).
McManus, M.J., Murphy, M.P. & Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 15703–15715 (2011).
Lowes, D.A., Webster, N.R., Murphy, M.P. & Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 110, 472–480 (2013).
Wipf, P. et al. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J. Am. Chem. Soc. 127, 12460–12461 (2005).
Xun, Z. et al. Targeting of XJB-5–131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington's disease. Cell Rep. 2, 1137–1142 (2012).
DeNicola, G.M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Maddocks, O.D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009).
Pei, S. et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J. Biol. Chem. doi:10.1074/jbc.M113.511170 (2013).
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by b-phenylethyl isothiocyanate. Cancer Cell 10, 241–252 (2006).
Raj, L. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475, 231–234 (2011).
Dolma, S., Lessnick, S.L., Hahn, W.C. & Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009).
Kohanski, M.A., Dwyer, D.J., Hayete, B., Lawrence, C.A. & Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007).
Liu, Y. & Imlay, J.A. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339, 1210–1213 (2013).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Cochemé, H.M. et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 (2011).
Albrecht, S.C., Barata, A.G., Grosshans, J., Teleman, A.A. & Dick, T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 (2011).
Au-Yeung, H.Y., Chan, J., Chantarojsiri, T. & Chang, C.J. Molecular imaging of labile iron(II) pools in living cells with a turn-on fluorescent probe. J. Am. Chem. Soc. 135, 15165–15173 (2013).
Acknowledgements
This work was supported by a K99 Pathway to Independence Award from the National Cancer Institute to S.J.D. (1K99CA166517-01). B.R.S. is an Early Career Scientist of the Howard Hughes Medical Institute and is supported by grants from the US National Institutes of Health (5R01CA097061, 5R01GM085081 and R01CA161061) and New York State Stem Cell Science.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dixon, S., Stockwell, B. The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10, 9–17 (2014). https://doi.org/10.1038/nchembio.1416
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1416
This article is cited by
-
Ferroptosis: a promising candidate for exosome-mediated regulation in different diseases
Cell Communication and Signaling (2024)
-
Methionine enkephalin (MENK) protected macrophages from ferroptosis by downregulating HMOX1 and ferritin
Proteome Science (2024)
-
Plasticity changes in iron homeostasis in hibernating Daurian ground squirrels (Spermophilus dauricus) may counteract chronically inactive skeletal muscle atrophy
Journal of Comparative Physiology B (2024)
-
Alleviated NCOA4-mediated ferritinophagy protected RA FLSs from ferroptosis in lipopolysaccharide-induced inflammation under hypoxia
Inflammation Research (2024)
-
Netrin-1 Alleviates Early Brain Injury by Regulating Ferroptosis via the PPARγ/Nrf2/GPX4 Signaling Pathway Following Subarachnoid Hemorrhage
Translational Stroke Research (2024)