Abstract
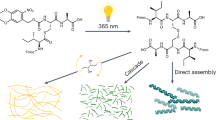
Through controlled synthesis and molecular assembly, biological systems are able to organize molecules into supramolecular structures that carry out sophisticated processes. Although chemists have reported a few examples of supramolecular assembly in water, the controlled covalent synthesis of large molecules and structures in vivo has remained challenging. Here we report a condensation reaction between 1,2-aminothiol and 2-cyanobenzothiazole that occurs in vitro and in living cells under the control of either pH, disulfide reduction or enzymatic cleavage. In vitro, the size and shape of the condensation products, and the nanostructures subsequently assembled, were different in each case and could thus be controlled by tuning the structure of the monomers. Direct imaging of the products obtained in the cells revealed their locations—near the Golgi bodies under enzymatic cleavage control—demonstrating the feasibility of a controlled and localized reaction in living cells. This intracellular condensation process enabled the imaging of the proteolytic activity of furin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 January 2010
In the version of this Article originally published, in Fig. 1b the groups labelled R1 and R2 were switched. This has now been corrected in the HTML and PDF versions of the Article.
References
Whitesides, G. M., Mathias, J. P. & Seto, C. T. Molecular self-assembly and nanochemistry: A chemical strategy for synthesis of nanostructure. Science 254, 1312–1319 (1991).
Lehn, J. M. Perspectives in supramolecular chemistry-from molecular recognition towards molecular information-processing and self-organization. Angew. Chem. Int. Ed. 29, 1304–1319 (1990).
Estroff, L. A. & Hamilton, A. D. Water gelation by small organic molecules. Chem. Rev. 104, 1201–1217 (2004).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Petka, W. A., Harden, J. L., McGrath, K. P., Wirtz, D. & Tirrell, D. A. Reversible hydrogels from self-assembling artificial proteins. Science 281, 389–392 (1998).
Yang, Z., Liang, G. & Xu, B. Enzymatic hydrolation of small molecules. Acc. Chem. Res. 41, 315–326 (2008).
Saxon, E. & Bertozzi, C. R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Laughlin, S. T., Baskin, J. M., Amacher, S. L. & Bertozzi, C. R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 (2008).
Kolb, H. C., Finn, M.G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Link, A. J. & Tirrell, D. A. Cell surface labeling of Escherichia coli via copper (I)-catalyzed [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 11164–11165 (2003).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Hsu, T.-L. et al. Alkynyl sugar analogs for labeling and visualization of glycoconjugates in cells. Proc. Natl Acad. Sci. USA 104, 2614–2619 (2007).
Mahal, L. K., Yarema, K. J. & Bertozzi, C. R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276, 1125–1128 (1997).
Zeng, Y., Ramya, T. N. C., Dirksen, A., Dawson, P. E. & Paulson, J. C. High-efficiency labeling of sialylated glycoproteins on living cells. Nature Methods 6, 207–209 (2009).
White, E. H., McCapra, F., Field, G. F. & McElroy, W. D. The structure and synthesis of firefly luciferin. J. Am. Chem. Soc. 83, 2402–2403 (1961).
Ren, H. et al. A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins. Angew. Chem. Int. Ed. doi:10.1002/anie.200903627 (2009).
Okada, K., Iio, H., Kubota, I. & Goto, T. Firefly bioluminescence III. Conversion of oxyluciferin to luciferin in firefly. Tetrahedron Lett. 32, 2771–2774 (1974).
Gomi, K. & Kajiyama, N. Oxyluciferin, a luminescence product of firefly luciferase, is enzymatically regenerated into luciferin. J. Biol. Chem. 276, 36508–36513 (2001).
Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3, 753–766 (2002).
Jagasia, R., Holub, J. M., Bollinger, M., Kirshenbaum, K. & Finn, M. G. Peptide cyclization and cylcodimerization by Cu+-mediated azide-alkyne cycloaddition. J. Org. Chem. 74, 2964–2974 (2009).
Punna, S., Kuzelka, J., Wang, Q. & Finn, M. G. Head-to-tail peptide cyclodimerization by copper-catalyzed azide-alkyne cycloaddition. Angew. Chem. Int. Ed. 44, 2215–2220 (2005).
Hosaka, M. et al. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin. J. Biol. Chem. 266, 12127–12130 (1991).
Talanian, R. V. et al. Substrate specificities of caspase family proteases. J. Biol. Chem. 272, 9677–9682 (1997).
Yang, Z. M., Xu, K. M., Guo, Z. F., Guo, Z. H. & Xu, B. Intracellular enzymatic formation of nanofibers results in hydrogelation and regulated cell death. Adv. Mater. 17, 3152–3156 (2007).
Shapiro, J. et al. Localization of endogenous furin in cultured cell lines. J. Histochem. Cytochem. 45, 3–12 (1997).
Henrich, S. et al. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nature Struct. Biol. 10, 520–526 (2003).
Bassi, D. E., Fu, J., de Cicco, R. L. & Klein-Szanto, A. J. P. Proprotein convertases: ‘Master switches’ in the regulation of tumor growth and progression. Mol. Carcinogen. 44, 151–161 (2005).
Cheng, M. et al. Pro-protein convertase gene expression in human breast cancer. Int. J. Cancer 71, 966–971(1997).
Tandon, A. K., Clark, G. M., Chamness, G. C., Chirgwin, J. M. & McGuire, W. L. Cathepsin-D and prognosis in breast cancer. New Eng. J. Med. 322, 297–302 (1990).
Yan, S. Q., Sameni, M. & Solane, B. F. Cathepsin B and human tumor progression. Biol. Chem. 379, 113–123 (1998).
Dragulescu-Andrasi, A., Liang, G. & Rao, J. In vivo bioluminescence imaging of furin activity in breast cancer cells using bioluminogenic substrates. Bioconjugate Chem. 20, 1660–1666 (2009).
Acknowledgements
The authors thank R. Chin at Stanford Nanocharacterization Laboratory for the technical assistance with TEM experiments, S. R. Lynch at the NMR Facility of Stanford Chemistry Department for help with the HMBC two-dimensional NMR experiment, J. Perrino at the Cell Sciences Imaging Facility of Stanford University for electron microscope imaging, and A. Dragulescu-Andrasi for help with the furin immunofluorescence staining. This work has been supported by a grant from NIGMS (R01GM086196-01) and an IDEA award from Department of Defense Breast Cancer Research Program (W81XWH-09-1-0057).
Author information
Authors and Affiliations
Contributions
G.L. and J.R. conceived and designed the experiments, G.L. and H.R. performed the experiments, G.L. and J.R. analysed the data, and G.L. and J.R. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Stanford University is filing patent protection on some of the results in this manuscript.
Supplementary information
Supplementary information
Supplementary information (PDF 1871 kb)
Rights and permissions
About this article
Cite this article
Liang, G., Ren, H. & Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nature Chem 2, 54–60 (2010). https://doi.org/10.1038/nchem.480
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.480
This article is cited by
-
Self-propelled assembly of nanoparticles with self-catalytic regulation for tumour-specific imaging and therapy
Nature Communications (2024)
-
Cell spheroid creation by transcytotic intercellular gelation
Nature Nanotechnology (2023)
-
Accelerating the prediction and discovery of peptide hydrogels with human-in-the-loop
Nature Communications (2023)
-
In situ self-assembly for cancer therapy and imaging
Nature Reviews Materials (2023)
-
Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics
Nature Communications (2023)