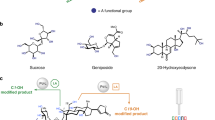
Abstract
The selective (and controllable) modification of complex molecules with disparate functional groups (for example, natural products) is a long-standing challenge that has been addressed using catalysts tuned to perform singular transformations (for example, C–H hydroxylation). A method whereby reactions with diverse functional groups within a single natural product are feasible depending on which catalyst or reagent is chosen would widen the possible structures one could obtain. Fluoroarylborane catalysts can heterolytically split Si–H bonds to yield an oxophilic silylium (R3Si+) equivalent along with a reducing (H–) equivalent. Together, these reactive intermediates enable the reduction of multiple functional groups. Exogenous phosphine Lewis bases further modify the catalyst speciation and attenuate aggressive silylium ions for the selective modification of complex natural products. Manipulation of the catalyst, silane reagent and the reaction conditions provides experimental control over which site is modified (and how). Applying this catalytic method to complex bioactive compounds (natural products or drugs) provides a powerful tool for studying structure–activity relationships.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wright, P. M., Seiple, I. B. & Myers, A. G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 53, 8840–8869 (2014).
Barnes, E. C., Kumar, R. & Davis, R. A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 33, 372–381 (2016).
Fischbach, M. A. & Walsh, C. T. Antibiotics for emerging pathogens. Science 325, 1089–1093 (2009).
Galloway, W. R. J. D., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010).
Lachance, H., Wetzel, S., Kumar, K. & Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 55, 5989–6001 (2012).
Clemons, P. A. et al. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl Acad. Sci. USA 107, 18787–18792 (2010).
Hann, M. M., Leach, A. R. & Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 41, 856–864 (2001).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist's toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Wilcock, B. C. et al. Electronic tuning of site-selectivity. Nat. Chem. 4, 996–1003 (2012).
Huigens, R. W. III et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nat. Chem. 5, 195–202 (2013).
He, J., Hamann, L. G., Davies, H. M. L. & Beckwith, R. E. J. Late-stage C–H functionalization of complex alkaloids and drug molecules via intermolecular rhodium-carbenoid insertion. Nat. Commun. 6, 5943 (2015).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science. 318, 783–787 (2007).
Lewis, C. A. & Miller, S. J. Site-selective derivatization and remodeling of erythromycin A by using simple peptide-based chiral catalysts. Angew. Chem. Int. Ed. 45, 5616–5619 (2006).
Balthaser, B. R., Maloney, M. C., Beeler, A. B., Porco, J. A. & Snyder, J. K. Remodelling of the natural product fumagillol employing a reaction discovery approach. Nat. Chem. 3, 969–973 (2011).
Galloway, W. R. J. D. & Spring, D. R. Is synthesis the main hurdle for the generation of diversity in compound libraries for screening? Expert Opin. Drug Discov. 4, 467–472 (2009).
Kim, K.-C. et al. Crystallographic evidence for a free silylium ion. Science 297, 825–827 (2002).
Reed, C. A. The silylium ion problem, R3Si+. Bridging organic and inorganic chemistry. Acc. Chem. Res. 31, 325–332 (1998).
Klare, H. F. T. & Oestreich, M. Silylium ions in catalysis. Dalton Trans. 39, 9176–9184 (2010).
Houghton, A. Y., Hurmalainen, J., Mansikkamäki, A., Piers, W. E. & Tuononen, H. M. Direct observation of a borane–silane complex involved in frustrated Lewis-pair-mediated hydrosilylations. Nat. Chem. 6, 983–988 (2014).
Rendler, S. & Oestreich, M. Conclusive evidence for an SN2-Si mechanism in the B(C6F5)3-catalyzed hydrosilylation of carbonyl compounds: implications for the related hydrogenation. Angew. Chem. Int. Ed. 47, 5997–6000 (2008).
Sakata, K. & Fujimoto, H. Quantum chemical study of B(C6F5)3-catalyzed hydrosilylation of carbonyl group. J. Org. Chem. 78, 12505–12512 (2013).
Chatterjee, I., Porwal, D. & Oestreich, M. B(C6F5)3-catalyzed chemoselective defunctionalization of ether-containing primary alkyl tosylates with hydrosilanes. Angew. Chem. Int. Ed. 56, 3389–3391 (2017).
Piers, W. E., Marwitz, A. J. V. & Mercier, L. G. Mechanistic aspects of bond activation with perfluoroarylboranes. Inorg. Chem. 50, 12252–12262 (2011).
Oestreich, M., Hermeke, J. & Mohr, J. A unified survey of Si–H and H–H bond activation catalysed by electron-deficient boranes. Chem. Soc. Rev. 44, 2202–2220 (2015).
Parks, D. J. & Piers, W. E. Tris(pentafluorophenyl)boron-catalyzed hydrosilation of aromatic aldehydes, ketones, and esters. J. Am. Chem. Soc. 118, 9440–9441 (1996).
Gevorgyan, V., Rubin, M., Benson, S., Liu, J.-X. & Yamamoto, Y. A novel B(C6F5)3-catalyzed reduction of alcohols and cleavage of aryl and alkyl ethers with hydrosilanes. J. Org. Chem. 65, 6179–6186 (2000).
Drosos, N. & Morandi, B. Boron-catalyzed regioselective deoxygenation of terminal 1,2-diols to 2-alkanols enabled by the strategic formation of a cyclic siloxane intermediate. Angew. Chem. Int. Ed. 54, 8814–8818 (2015).
Drosos, N., Ozkal, E. & Morandi, B. Catalytic selective deoxygenation of polyols using the B(C6F5)3/silane system. Synlett 27, 1760–1764 (2016).
Gandhamsetty, N., Park, S. & Chang, S. Selective silylative reduction of pyridines leading to structurally diverse azacyclic compounds with the formation of sp3 C–Si bonds. J. Am. Chem. Soc. 137, 15176–15184 (2015).
Gandhamsetty, N. et al. Chemoselective silylative reduction of conjugated nitriles under metal-free catalytic conditions: β-silyl amines and enamines. Angew. Chem. Int. Ed. 54, 6832–6836 (2015).
Chandrasekhar, S., Chandrashekar, G., Reddy, M. S. & Srihari, P. A facile and chemoselective conjugate reduction using polymethylhydrosiloxane (PMHS) and catalytic B(C6F5)3 . Org. Biomol. Chem. 4, 1650–1652 (2006).
Tiddens, M. R., Klein Gebbink, R. J. M. & Otte, M. The B(C6F5)3-catalyzed tandem Meinwald rearrangement–reductive amination. Org. Lett. 18, 3714–3717 (2016).
Mack, D. J., Guo, B. & Njardarson, J. T. Synthesis of allylic and homoallylic alcohols from unsaturated cyclic ethers using a mild and selective C–O reduction approach. Chem. Commun. 48, 7844–7846 (2012).
Zhang, Q., Fu, M.-C., Yu, H.-Z. & Fu, Y. Mechanism of boron-catalyzed N-alkylation of amines with carboxylic acids. J. Org. Chem. 81, 6235–6243 (2016).
Porwal, D. & Oestreich, M. B(C6F5)3-catalyzed reduction of aromatic and aliphatic nitro groups with hydrosilanes. Eur. J. Org. Chem. 2016, 3307–3309 (2016).
Lee, P. T. K. & Rosenberg, L. Scope and selectivity of B(C6F5)3-catalyzed reactions of the disilane (Ph2SiH)2 . J. Organomet. Chem. 809, 86–93 (2016).
Huang, P.-Q., Lang, Q.-W. & Wang, Y.-R. Mild metal-free hydrosilylation of secondary amides to amines. J. Org. Chem. 81, 4235–4243 (2016).
Adduci, L. L., Bender, T. A., Dabrowski, J. A. & Gagné, M. R. Chemoselective conversion of biologically sourced polyols into chiral synthons. Nat. Chem. 7, 576–581 (2015).
Bender, T. A., Dabrowski, J. A., Zhong, H. & Gagné, M. R. Diastereoselective B(C6F5)3-catalyzed reductive carbocyclization of unsaturated carbohydrates. Org. Lett. 18, 4120–4123 (2016).
Bender, T. A., Dabrowski, J. A. & Gagné, M. R. Delineating the multiple roles of B(C6F5)3 in the chemoselective deoxygenation of unsaturated polyols. ACS Catal. 6, 8399–8403 (2016).
Palacios, D. S., Dailey, I., Siebert, D. M., Wilcock, B. C. & Burke, M. D. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl Acad. Sci. USA 108, 6733–6738 (2011)
Volmer, A. A., Szpilman, A. M. & Carreira, E. M. Synthesis and biological evaluation of amphotericin B derivatives. Nat. Prod. Rep. 27, 1329–1349 (2010).
Palacios, D. S., Anderson, T. M. & Burke, M. D. A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J. Am. Chem. Soc. 129, 13804–13805 (2007).
Shimano, M., Nagaoka, H. & Yamada, Y. Synthesis of gibberellin A1, A5. A55 and A60. Metal–ammonia reduction of gibberellic acid and its derivatives. Chem. Pharm. Bull. 38, 276–278 (1990).
Soomro, S. et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 15, 1122–1135 (2011).
Heiden, Z. M. & Lathem, A. P. Establishing the hydride donor abilities of main group hydrides. Organometallics 34, 1818–1827 (2015).
Kim, Y. & Chang, S. Borane-catalyzed reductive α-silylation of conjugated esters and amides leaving carbonyl groups intact. Angew. Chem. Int. Ed. 55, 218–222 (2016).
Hoveyda, A. H., Evans, D. A. & Fu, G. C. Substrate-directable chemical reactions. Chem. Rev. 93, 1307–1370 (1993).
Simmons, E. M. & Hartwig, J. F. Catalytic functionalization of unactivated primary C–H bonds directed by an alcohol. Nature 483, 70–73 (2012).
Stephan, D. W. & Erker, G. Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew. Chem. Int. Ed. 49, 46–76 (2009).
Chen, D., Leich, V., Pan, F. & Klankermayer, J. Enantioselective hydrosilylation with chiral frustrated Lewis pairs. Chem. Eur. J. 18, 5184–5187 (2012).
Parks, D. J., von H. Spence, R. E. & Piers, W. E. Bis(pentafluorophenyl)borane: synthesis, properties, and hydroboration chemistry of a highly electrophilic borane reagent. Angew. Chem. Int. Ed. 34, 809–811 (1995).
Volkov, A., Tinnis, F., Slagbrand, T., Trillo, P. & Adolfsson, H. Chemoselective reduction of carboxamides. Chem. Soc. Rev. 45, 6685–6697 (2016).
Mukherjee, D., Shirase, S., Mashima, K. & Okuda, J. Chemoselective reduction of tertiary amides to amines catalyzed by triphenylborane. Angew. Chem. Int. Ed. 55, 13326–13329 (2016).
Kingston, D. G. I. The shape of things to come: structural and synthetic studies of taxol and related compounds. Phytochemistry 68, 1844–1854 (2007).
Gao, F., Yu, M., Chen, Q.-H. & Wang, F.-P. A selective intramolecular transacylation of taxoids accompanying with the oxetane ring opening. Chem. Pharm. Bull. 60, 415–418 (2012).
Acknowledgements
This work was exclusively supported by the Department of Energy (Basic Energy Sciences, DE-FG02-05ER15630). T.A.B. is grateful for a University of North Carolina Dissertation Completion Fellowship.
Author information
Authors and Affiliations
Contributions
T.A.B. and M.R.G. conceived and designed the experiments; T.A.B. performed the experiments; P.R.P. prepared Lewis acid catalysts; T.A.B. and M.R.G. participated in the process of data analysis and the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 18933 kb)
Rights and permissions
About this article
Cite this article
Bender, T., Payne, P. & Gagné, M. Late-stage chemoselective functional-group manipulation of bioactive natural products with super-electrophilic silylium ions. Nature Chem 10, 85–90 (2018). https://doi.org/10.1038/nchem.2863
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2863
This article is cited by
-
Direct nucleophilic and electrophilic activation of alcohols using a unified boron-based organocatalyst scaffold
Nature Communications (2023)
-
Iridium and B(C6F5)3 co-catalyzed chemoselective deoxygenative reduction of tertiary amides: application to the efficient synthesis and late-stage modification of pharmaceuticals
Science China Chemistry (2023)