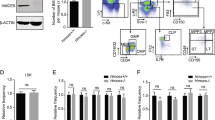
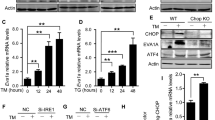
Abstract
BID, a BH3-only BCL2 family member, functions in apoptosis as well as the DNA-damage response1. Our previous data demonstrated that BID is an ATM effector acting to induce cell-cycle arrest and inhibition of apoptosis following DNA damage2,3. Here we show that ATM-mediated BID phosphorylation plays an unexpected role in maintaining the quiescence of haematopoietic stem cells (HSCs). Loss of BID phosphorylation leads to escape from quiescence of HSCs, resulting in exhaustion of the HSC pool and a marked reduction of HSC repopulating potential in vivo. We also demonstrate that BID phosphorylation plays a role in protecting HSCs from irradiation, and that regulating both quiescence and survival of HSCs depends on BID’s ability to regulate oxidative stress. Moreover, loss of BID phosphorylation, ATM knockout or exposing mice to irradiation leads to an increase in mitochondrial BID, which correlates with an increase in mitochondrial oxidative stress. These results show that the ATM–BID pathway serves as a critical checkpoint for coupling HSC homeostasis and the DNA-damage stress response to enable long-term regenerative capacity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yin, X. M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999).
Kamer, I. et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 122, 593–603 (2005).
Zinkel, S. S. et al. A role for proapoptotic BID in the DNA-damage response. Cell 122, 579–591 (2005).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Lavin, M. F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 9, 759–769 (2008).
Bartek, J., Lukas, C. & Lukas, J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 5, 792–804 (2004).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451 (2006).
Whetton, A. D. Stem cells bank on ATM machine. Nat. Med. 10, 1166–1168 (2004).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Celeste, A. et al. Genomic instability in mice lacking histone H2AX. Science 296, 922–927 (2002).
Ward, I. M., Minn, K., van Deursen, J. & Chen, J. p53 binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol. Cell Biol. 23, 2556–2563 (2003).
Orford, K. W. & Scadden, D. T. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet 9, 115–128 (2008).
Liu, J. et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459, 387–392 (2009).
Molofsky, A. V. et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 (2003).
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003).
Mukhopadhyay, P. et al. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2, 2295–2301 (2007).
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Gan, B. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704 (2010).
Gurumurthy, S. et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659–663 (2010).
Hock, H. et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431, 1002–1007 (2004).
Nakada, D., Saunders, T. L. & Morrison, S. J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653–658 (2010).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Zhang, J. et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 (2006).
Jang, Y. Y. & Sharkis, S. J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063 (2007).
Parmar, K., Mauch, P., Vergilio, J. A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007).
Tesio, M. et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117, 419–428 (2011).
Juntilla, M. M. et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 (2010).
Zaltsman, Y. et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell Biol. 12, 553–562 (2010).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet 41, 25–34 (2009).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Andersen, J. S. et al. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12, 1–11 (2002).
Mihara, M. & Moll, U. M. Detection of mitochondrial localization of p53. Methods Mol. Biol. 234, 203–209 (2003).
Grinberg, M. et al. Mitochondrial carrier homolog 2 is a target of tBID in cells signaled to die by tumor necrosis factor alpha. Mol. Cell Biol. 25, 4579–4590 (2005).
George, T. C. et al. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J. Immunol. Methods 311, 117–129 (2006).
Acknowledgements
We are grateful to A. Harmelin, R. Haffner, A. Maizenberg, G. Damari, Y. Chermesh, C. Raanan and N. Nevo for help with the animal studies, and I. Ino, O. Amram and E. Gamil for animal maintenance. We are especially grateful to Y. Shilo (Tel Aviv University) for ATM−/− mice, and to T. Lapidot (Weizmann Institute) for advice and for reading the manuscript. We also thank A. Sharp, E. Ariel and Z. Porat for assistance with flow-cytometric studies and cell sorting and J. Lotem for advice regarding some of the studies. This study was supported in part by the Israel Science Foundation, USA–Israel Binational Science Foundation, German–Israel Foundation, German–Israel Research Program in Cancer Research, Israel Cancer Association, Minerva Stiftung and MDM ICR Research Award. A.G. is the incumbent of the Armour Family Career Development Chair of Cancer Research.
Author information
Authors and Affiliations
Contributions
M.M. carried out most of the experiments presented in the paper. G.O. carried out some of the initial experiments. H.N. generated the BIDAA mice and carried out the initial characterization of these mice. L.V. carried out the BID−/− and γH2AX studies, and Y.Z. helped with many of the experiments and especially with the subcellular fractionation studies. O.B. carried out the pathology of bone-marrow sections, T.L. was our consultant on HSC biology and S.J. was our main consultant on all the immunology-related studies. M.M. and A.G. planned the projects and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1370 kb)
Rights and permissions
About this article
Cite this article
Maryanovich, M., Oberkovitz, G., Niv, H. et al. The ATM–BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol 14, 535–541 (2012). https://doi.org/10.1038/ncb2468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2468
This article is cited by
-
Role of Caspase-10-P13tBID axis in erythropoiesis regulation
Cell Death & Differentiation (2023)
-
Cell-intrinsic factors governing quiescence vis-à-vis activation of adult hematopoietic stem cells
Molecular and Cellular Biochemistry (2023)
-
The Dual Role of ROS in Hematological Malignancies: Stem Cell Protection and Cancer Cell Metastasis
Stem Cell Reviews and Reports (2020)
-
Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche
Nature Biomedical Engineering (2020)
-
Diminished apoptotic priming and ATM signalling confer a survival advantage onto aged haematopoietic stem cells in response to DNA damage
Nature Cell Biology (2018)