Abstract
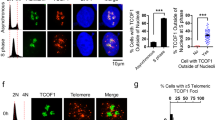
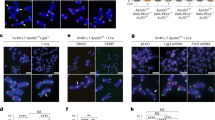
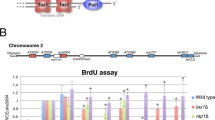
Kinases and phosphatases regulate messenger RNA synthesis through post-translational modification of the carboxy-terminal domain (CTD) of the largest subunit of RNA polymerase II (ref. 1). In yeast, the phosphatase Cdc14 is required for mitotic exit2,3 and for segregation of repetitive regions4. Cdc14 is also a subunit of the silencing complex RENT (refs 5, 6), but no roles in transcriptional repression have been described. Here we report that inactivation of Cdc14 causes silencing defects at the intergenic spacer sequences of ribosomal genes during interphase and at Y′ repeats in subtelomeric regions during mitosis. We show that the role of Cdc14 in silencing is independent of the RENT deacetylase subunit Sir2. Instead, Cdc14 acts directly on RNA polymerase II by targeting CTD phosphorylation at Ser 2 and Ser 5. We also find that the role of Cdc14 as a CTD phosphatase is conserved in humans. Finally, telomere segregation defects in cdc14 mutants4 correlate with the presence of subtelomeric Y′ elements and can be rescued by transcriptional inhibition of RNA polymerase II.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Orphanides, G. & Reinberg, D. A unified theory of gene expression. Cell 108, 439–451 (2002).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 2, 709–718 (1998).
Stegmeier, F. & Amon, A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38, 203–232 (2004).
D’Amours, D., Stegmeier, F. & Amon, A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell 117, 455–469 (2004).
Shou, W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–244 (1999).
Visintin, R., Hwang, E. S. & Amon, A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398, 818–823 (1999).
Stegmeier, F., Visintin, R. & Amon, A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207–220 (2002).
Torres-Rosell, J., Machin, F., Jarmuz, A. & Aragon, L. Nucleolar segregation lags behind the rest of the genome and requires Cdc14p activation by the FEAR network. Cell Cycle 3, 496–502 (2004).
Sullivan, M., Higuchi, T., Katis, V. L. & Uhlmann, F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell 117, 471–482 (2004).
Machin, F. et al. Transcription of ribosomal genes can cause nondisjunction. J. Cell Biol. 173, 893–903 (2006).
Tomson, B. N., D’Amours, D., Adamson, B. S., Aragon, L. & Amon, A. Ribosomal DNA transcription-dependent processes interfere with chromosome segregation. Mol. Cell. Biol. 26, 6239–6247 (2006).
Clemente-Blanco, A. et al. Cdc14 inhibits transcription by RNA polymerase I during anaphase. Nature 458, 219–222 (2009).
Smith, J. S. & Boeke, J. D. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11, 241–254 (1997).
Bryk, M. et al. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11, 255–269 (1997).
Straight, A. F. et al. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97, 245–256 (1999).
Imai, S. et al. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb. Symp. Quant. Biol. 65, 297–302 (2000).
Houseley, J., Kotovic, K., El Hage, A. & Tollervey, D. Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J. 26, 4996–5006 (2007).
Egloff, S. & Murphy, S. Cracking the RNA polymerase II CTD code. Trends Genet. 24, 280–288 (2008).
Mayan, M. & Aragon, L. Cis-interactions between non-coding ribosomal spacers dependent on RNAP-II separate RNAP-I and RNAP-III transcription domains. Cell Cycle 9, 4328–4337 (2010).
Jaspersen, S. L. & Morgan, D. O. Cdc14 activates cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 10, 615–618 (2000).
Jiang, Y. Regulation of the cell cycle by protein phosphatase 2A in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 440–449 (2006).
Doi, K. et al. MSG5, a novel protein phosphatase promotes adaptation to pheromone response in S. cerevisiae. EMBO J. 13, 61–70 (1994).
Krishnamurthy, S., He, X., Reyes-Reyes, M., Moore, C. & Hampsey, M. Ssu72 is an RNA polymerase II CTD phosphatase. Mol. Cell 14, 387–394 (2004).
David, L. et al. A high-resolution map of transcription in the yeast genome. Proc. Natl Acad. Sci. USA 103, 5320–5325 (2006).
Wang, B. D., Butylin, P. & Strunnikov, A. Condensin function in mitotic nucleolar segregation is regulated by rDNA transcription. Cell Cycle 5, 2260–2267 (2006).
Freeman, L., Aragon-Alcaide, L. & Strunnikov, A. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 149, 811–824 (2000).
Cuylen, S., Metz, J. & Haering, C. H. Condensin structures chromosomal DNA through topological links. Nat. Struct. Mol. Biol. 18, 894–901 (2011).
Lengronne, A. et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430, 573–578 (2004).
Chan, J. N. et al. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev. Cell 20, 867–879 (2011).
Dulev, S. et al. Essential global role of CDC14 in DNA synthesis revealed by chromosome underreplication unrecognized by checkpoints in cdc14 mutants. Proc. Natl Acad. Sci. USA 106, 14466–14471 (2009).
D’Ambrosio, C., Kelly, G., Shirahige, K. & Uhlmann, F. Condensin-dependent rDNA decatenation introduces a temporal pattern to chromosome segregation. Curr. Biol. 18, 1084–1089 (2008).
Baxter, J. et al. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 331, 1328–1332 (2011).
Chafin, D. R., Guo, H. & Price, D. H. Action of α-amanitin during pyrophosphorolysis and elongation by RNA polymerase II. J. Biol. Chem. 270, 19114–19119 (1995).
Bembenek, J & Yu, H Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J. Biol. Chem. 276, 48237–48242 (2001).
Mailand, N. et al. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat. Cell Biol. 4, 317–322 (2002).
Paulsen, M. T. et al. The p53-targeting human phosphatase hCdc14A interacts with the Cdk1/cyclin B complex and is differentially expressed in human cancers. Mol. Cancer 5, 25 (2006).
Kaiser, B. K., Zimmerman, Z. A., Charbonneau, H. & Jackson, P. K. Disruption of centrosome structure, chromosome segregation, and cytokinesis by misexpression of human Cdc14A phosphatase. Mol. Biol. Cell 13, 2289–2300 (2002).
Bassermann, F. et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134, 256–267 (2008).
Acknowledgements
We are very grateful to L. Warfield and S. Hahn for advice. We thank D. Morgan (UCSF) and A. Amon (MIT) for reagents. We are grateful to all members of the Aragon laboratory for discussions and comments on the manuscript. The Bueno and Sacristán laboratories are financially supported by grants from the Spanish Science Ministry (MICINN). The Eick laboratory is supported by the Deutsche Forschungsgemeinschaft, SFB-Transregio5. The Aragon and Merkenschlager laboratories are supported by the Medical Research Council (MRC) of the UK.
Author information
Authors and Affiliations
Contributions
A.C-B. and L.A. conceived the study and analysed the data with critical input from M.M. Experiments were conducted by A.C-B., N.S., M.M-S., A.J. and B.G. Microarray analysis was done by L.G. A.G. and E.W. Critical materials were provided by M.P.S., A.B. and D.E. L.A. wrote the paper and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1453 kb)
Rights and permissions
About this article
Cite this article
Clemente-Blanco, A., Sen, N., Mayan-Santos, M. et al. Cdc14 phosphatase promotes segregation of telomeres through repression of RNA polymerase II transcription. Nat Cell Biol 13, 1450–1456 (2011). https://doi.org/10.1038/ncb2365
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2365
This article is cited by
-
Biochemical analyses reveal amino acid residues critical for cell cycle-dependent phosphorylation of human Cdc14A phosphatase by cyclin-dependent kinase 1
Scientific Reports (2018)
-
The loading of condensin in the context of chromatin
Current Genetics (2017)
-
Transcription-replication conflicts at chromosomal fragile sites—consequences in M phase and beyond
Chromosoma (2017)
-
Specific threonine-4 phosphorylation and function of RNA polymerase II CTD during M phase progression
Scientific Reports (2016)
-
The pol II CTD: new twists in the tail
Nature Structural & Molecular Biology (2016)