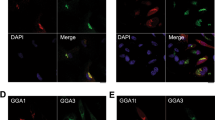
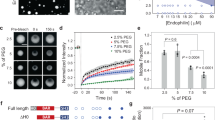
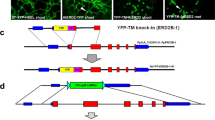
Abstract
Endocytic sorting of signalling receptors between recycling and degradative pathways is a key cellular process controlling the surface complement of receptors and, accordingly, the cell’s ability to respond to specific extracellular stimuli. The β2 adrenergic receptor (β2AR) is a prototypical seven-transmembrane signalling receptor that recycles rapidly and efficiently to the plasma membrane after ligand-induced endocytosis. β2AR recycling is dependent on the receptor’s carboxy-terminal PDZ ligand and Rab4 (refs 1, 2). This active sorting process is required for functional resensitization of β2AR-mediated signalling3,4. Here we show that sequence-directed sorting occurs at the level of entry into retromer tubules and that retromer tubules are associated with Rab4. Furthermore, we show that sorting nexin 27 (SNX27) serves as an essential adaptor protein linking β2ARs to the retromer tubule. SNX27 does not seem to directly interact with the retromer core complex, but does interact with the retromer-associated Wiskott–Aldrich syndrome protein and SCAR homologue (WASH) complex. The present results identify a role for retromer in endocytic trafficking of signalling receptors, in regulating a receptor-linked signalling pathway, and in mediating direct endosome-to-plasma membrane traffic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Seachrist, J. L., Anborgh, P. H. & Ferguson, S. S. β2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 275, 27221–27228 (2000).
Cao, T. T., Deacon, H. W., Reczek, D., Bretscher, A. & von Zastrow, M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401, 286–290 (1999).
Wang, Y., Lauffer, B., Von Zastrow, M., Kobilka, B. K. & Xiang, Y. N-ethylmaleimide-sensitive factor regulates β2 adrenoceptor trafficking and signaling in cardiomyocytes. Mol. Pharmacol. 72, 429–439 (2007).
Hanyaloglu, A. C. & von Zastrow, M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 (2008).
Pippig, S., Andexinger, S. & Lohse, M. J. Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 47, 666–676 (1995).
Maxfield, F. R. & McGraw, T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132 (2004).
Puthenveedu, M. A. et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143, 761–773 (2010).
Sonnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J. & Zerial, M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 (2000).
Carlton, J. G. & Cullen, P. J. Sorting nexins. Curr. Biol. 15, R819–R820 (2005).
Carlton, J. et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14, 1791–1800 (2004).
Mari, M. et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9, 380–393 (2008).
Bonifacino, J. S. & Hurley, J. H. Retromer. Curr. Opin. Cell Biol. 20, 427–436 (2008).
Rojas, R. et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 183, 513–526 (2008).
Bonifacino, J. S. & Rojas, R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7, 568–579 (2006).
Mayor, S., Presley, J. F. & Maxfield, F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 121, 1257–1269 (1993).
Seaman, M. N. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165, 111–122 (2004).
Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R. & Bonifacino, J. S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123–133 (2004).
Moore, R. H., Millman, E. E., Alpizar-Foster, E., Dai, W. & Knoll, B. J. Rab11 regulates the recycling and lysosome targeting of β2-adrenergic receptors. J. Cell Sci. 117, 3107–3117 (2004).
von Zastrow, M. & Kobilka, B. K. Ligand-regulated internalization and recycling of human β2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J. Biol. Chem. 267, 3530–3538 (1992).
Lin, S. X., Mallet, W. G., Huang, A. Y. & Maxfield, F. R. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol. Biol. Cell 15, 721–733 (2004).
Roth, J. & Berger, E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J. Cell Biol. 93, 223–229 (1982).
Cole, N. B. et al. Diffusional mobility of Golgi proteins in membranes of living cells. Science 273, 797–801 (1996).
Franch-Marro, X. et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat. Cell Biol. 10, 170–177 (2008).
Mallard, F. et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653–664 (2002).
Ganley, I. G., Espinosa, E. & Pfeffer, S. R. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J. Cell Biol. 180, 159–172 (2008).
Progida, C. et al. Rab7b controls trafficking from endosomes to the TGN. J. Cell Sci. 123, 1480–1491 (2010).
Derby, M. C. et al. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic 8, 758–773 (2007).
Utskarpen, A., Slagsvold, H. H., Iversen, T. G., Walchli, S. & Sandvig, K. Transport of ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A′. Traffic 7, 663–672 (2006).
Seaman, M. N. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 120, 2378–2389 (2007).
Lauffer, B. E. et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565–574 (2010).
Gomez, T. S. & Billadeau, D. D. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev. Cell 17, 699–711 (2009).
He, J. et al. Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J. Biol. Chem. 281, 2820–2827 (2006).
Gage, R. M., Matveeva, E. A., Whiteheart, S. W. & von Zastrow, M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J. Biol. Chem. 280, 3305–3313 (2005).
Vargas, G. A. & Von Zastrow, M. Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J. Biol. Chem. 279, 37461–37469 (2004).
Heydorn, A. et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J. Biol. Chem. 279, 54291–54303 (2004).
Verges, M. et al. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 6, 763–769 (2004).
Tabuchi, M., Yanatori, I., Kawai, Y. & Kishi, F. Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. J. Cell Sci. 123, 756–766 (2010).
Kleine-Vehn, J. et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl Acad. Sci. USA 105, 17812–17817 (2008).
Strochlic, T. I., Setty, T. G., Sitaram, A. & Burd, C. G. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J. Cell Biol. 177, 115–125 (2007).
Marchese, A., Paing, M. M., Temple, B. R. & Trejo, J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 48, 601–629 (2008).
Holmes, K. D., Babwah, A. V., Dale, L. B., Poulter, M. O. & Ferguson, S. S. Differential regulation of corticotropin releasing factor 1α receptor endocytosis and trafficking by β-arrestins and Rab GTPases. J. Neurochem. 96, 934–949 (2006).
Tang, Y. et al. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the β1-adrenergic receptor. Proc. Natl Acad. Sci. USA 96, 12559–12564 (1999).
Jager, S. et al. Purification and characterization of HIV-human protein complexes. Methods 53, 13–19 (2011).
Cottrell, G. S. et al. Endosomal endothelin-converting enzyme-1: a regulator of β-arrestin-dependent ERK signaling. J. Biol. Chem. 284, 22411–22425 (2009).
Acknowledgements
We thank B. Padilla, J. Bonifacino, Y. Prabhu, N. Gulbahce, S. Duleh, M. Welch, R. Rojas, J. Hislop, M. Puthenveedu, K. Mostov, J. Weissman, K. Thorn, the Nikon Imaging Center, A. Burlingame and the UCSF Mass Spectrometry facility (supported by P41RR001614) for reagents, advice, technical training and support. Last, we thank H. Bourne and M. Ray for critical readings of the manuscript. This work was supported by research grants from the National Institutes of Health. P.T. was supported by the National Science Foundation. N.J.K. is a Searle and Keck Young Investigator Fellow.
Author information
Authors and Affiliations
Contributions
P.T. and M.v.Z. conceived the project and wrote the manuscript. P.T. carried out most of the experiments with contributions from B.L. Mass spectrometry was carried out by S.J., analysed by P.C., in the laboratory of N.J.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 379 kb)
Supplementary Information
Supplementary Movie 1 (MOV 1674 kb)
Supplementary Information
Supplementary Table 1 (XLS 20 kb)
Rights and permissions
About this article
Cite this article
Temkin, P., Lauffer, B., Jäger, S. et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13, 715–721 (2011). https://doi.org/10.1038/ncb2252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2252
This article is cited by
-
Profiling the proximal proteome of the activated μ-opioid receptor
Nature Chemical Biology (2024)
-
Endosome positioning coordinates spatially selective GPCR signaling
Nature Chemical Biology (2024)
-
A Rab10–ACAP1–Arf6 GTPases cascade modulates M4 muscarinic acetylcholine receptor trafficking and signaling
Cellular and Molecular Life Sciences (2023)
-
EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers
Oncogene (2022)
-
Metabolic reprogramming in astrocytes results in neuronal dysfunction in intellectual disability
Molecular Psychiatry (2022)