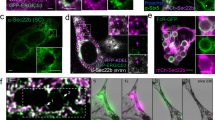
Abstract
During apoptosis, dying cells are swiftly removed by phagocytes. It is not fully understood how apoptotic cells are recognized by phagocytes. Here we report the identification and characterization of the Caenorhabditis elegans ttr-52 gene, which encodes a transthyretin-like protein and is required for efficient cell corpse engulfment. The TTR-52 protein is expressed in, and secreted from, C. elegans endoderm and clusters around apoptotic cells. Genetic analysis indicates that TTR-52 acts in the cell corpse engulfment pathway mediated by CED-1, CED-6 and CED-7 and affects clustering of the phagocyte receptor CED-1 around apoptotic cells. TTR-52 recognizes surface-exposed phosphatidylserine (PtdSer) in vivo and binds to both PtdSer and the extracellular domain of CED-1 in vitro. TTR-52 is therefore the first bridging molecule identified in C. elegans that mediates recognition of apoptotic cells by crosslinking the PtdSer 'eat me' signal with the phagocyte receptor CED-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 (2002).
Henson, P. M., Bratton, D. L. & Fadok, V. A. Apoptotic cell removal. Curr. Biol. 11, R795–R805 (2001).
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Reddien, P. W. & Horvitz, H. R. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 20, 193–221 (2004).
Reddien, P. W. & Horvitz, H. R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2, 131–136 (2000).
Wu, Y. C. & Horvitz, H. R. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392, 501–504 (1998).
Gumienny, T. L. et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107, 27–41 (2001).
Zhou, Z., Caron, E., Hartwieg, E., Hall, A. & Horvitz, H. R. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev. Cell 1, 477–489 (2001).
Wu, Y. C., Tsai, M. C., Cheng, L. C., Chou, C. J. & Weng, N. Y. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev. Cell 1, 491–502 (2001).
Zhou, Z., Hartwieg, E. & Horvitz, H. R. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104, 43–56 (2001).
Su, H. P. et al. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J. Biol. Chem. 277, 11772–11779 (2002).
Hamon, Y. et al. Cooperation between engulfment receptors: the case of ABCA1 and MEGF10. PLoS ONE 1, e120 (2006).
Manaka, J. et al. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J. Biol. Chem. 279, 48466–48476 (2004).
Kurant, E., Axelrod, S., Leaman, D. & Gaul, U. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell 133, 498–509 (2008).
MacDonald, J. M. et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50, 869–881 (2006).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Gardai, S. J., Bratton, D. L., Ogden, C. A. & Henson, P. M. Recognition ligands on apoptotic cells: a perspective. J. Leukoc. Biol. 79, 896–903 (2006).
Wang, X. et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nature Cell Biol. 9, 541–549 (2007).
Zullig, S. et al. Aminophospholipid translocase TAT-1 promotes phosphatidylserine exposure during C. elegans apoptosis. Curr. Biol. 17, 994–999 (2007).
Venegas, V. & Zhou, Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol. Biol. Cell 18, 3180–3192 (2007).
Darland-Ransom, M. et al. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science 320, 528–531 (2008).
Wang, X. et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science 302, 1563–1566 (2003).
Yu, X., Odera, S., Chuang, C. H., Lu, N. & Zhou, Z. C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev. Cell 10, 743–757 (2006).
Hoeppner, D. J., Hengartner, M. O. & Schnabel, R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412, 202–206 (2001).
Reddien, P. W., Cameron, S. & Horvitz, H. R. Phagocytosis promotes programmed cell death in C. elegans. Nature 412, 198–202 (2001).
Schreiber, G. The evolutionary and integrative roles of transthyretin in thyroid hormone homeostasis. J. Endocrinol. 175, 61–73 (2002).
Sonnhammer, E. L. & Durbin, R. Analysis of protein domain families in Caenorhabditis elegans. Genomics 46, 200–216 (1997).
Saverwyns, H. et al. Analysis of the transthyretin-like (TTL) gene family in Ostertagia ostertagi — comparison with other strongylid nematodes and Caenorhabditis elegans. Int. J. Parasitol. 38, 1545–1556 (2008).
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829 (1986).
Conradt, B. & Horvitz, H. R. The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98, 317–327 (1999).
Kennedy, B. P. et al. The gut esterase gene (ges-1) from the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. J. Mol. Biol. 229, 890–908 (1993).
Robertson, A. G. & Thomson, J. N. Morphology of programmed cell death in the ventral nerve chord of C. elegans larvae. J. Embryol. Exp. Morphol. 67, 89 (1982).
Sulston, J. E., Schierenberg, E., White, J. G. & Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 (1983).
Yeung, T. et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 (2008).
Shi, J., Heegaard, C. W., Rasmussen, J. T. & Gilbert, G. E. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim. Biophys. Acta 1667, 82–90 (2004).
Kritikou, E. A. et al. C. elegans GLA-3 is a novel component of the MAP kinase MPK-1 signaling pathway required for germ cell survival. Genes Dev. 20, 2279–2292 (2006).
Savill, J., Hogg, N., Ren, Y. & Haslett, C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522 (1992).
Anderson, H. A. et al. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol. 4, 87–91 (2003).
Balasubramanian, K., Chandra, J. & Schroit, A. J. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of β2-glycoprotein I in macrophage recognition. J. Biol. Chem. 272, 31113–31117 (1997).
Ishimoto, Y., Ohashi, K., Mizuno, K. & Nakano, T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. (Tokyo) 127, 411–417 (2000).
Vandivier, R. W. et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169, 3978–3986 (2002).
Savill, J., Fadok, V., Henson, P. & Haslett, C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today 14, 131–136 (1993).
Gardai, S. J. et al. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115, 13–23 (2003).
Eneqvist, T., Lundberg, E., Nilsson, L., Abagyan, R. & Sauer-Eriksson, A. E. The transthyretin-related protein family. Eur. J. Biochem. 270, 518–532 (2003).
Lundberg, E., Backstrom, S., Sauer, U. H. & Sauer-Eriksson, A. E. The transthyretin-related protein: structural investigation of a novel protein family. J. Struct. Biol. 155, 445–457 (2006).
Lee, Y. et al. Mouse transthyretin-related protein is a hydrolase which degrades 5-hydroxyisourate, the end product of the uricase reaction. Mol. Cells 22, 141–145 (2006).
Nam, K. H. & Li, J. The Arabidopsis transthyretin-like protein is a potential substrate of BRASSINOSTEROID-INSENSITIVE 1. Plant Cell 16, 2406–2417 (2004).
Fadeel, B. & Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277 (2009).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Riddle, D. L., Blumenthal, T., Meyer, B. J. & Preiss, J. R. (eds). C. elegans II (Cold Spring Harbor Laboratory Press, 1997).
Ellis, R. E., Jacobson, D. M. & Horvitz, H. R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129, 79–94 (1991).
Parrish, J. et al. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature 412, 90–94 (2001).
Geng, X. et al. Inhibition of CED-3 zymogen activation and apoptosis in Caenorhabditis elegans by caspase homolog CSP-3. Nat. Struct. Mol. Biol. 15, 1094–1101 (2008).
Acknowledgements
We thank J. McGhee for the Pges-1GFP construct and T. Blumenthal for comments and discussion on the manuscript. This work was supported by a Burroughs Wellcome Fund Award (D.X.), NIH R01 grants GM59083 and GM79097 (D.X.), and the National High Technology Project 863 of China (X.C.W).
Author information
Authors and Affiliations
Contributions
X.C.W. and W.D.L. performed most of the genetic and cell biological experiments. D.F.Z. performed both PtdSer binding experiments and in vitro protein interaction assays. Y.S. performed immunoprecipitation experiments in C. elegans. B.L., B.H.C., P.F.G. and X.G. performed some of the genetic and cell biological experiments. H.W.Y. performed the initial in vitro PtdSer binding experiments and E. P. conducted a bioinformatic analysis of TTR family proteins. Z.H.S., E.K.N. and S.M. contributed to the generation of strains. X.C.W. and D.X. designed the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 896 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Li, W., Zhao, D. et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol 12, 655–664 (2010). https://doi.org/10.1038/ncb2068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2068
This article is cited by
-
Defining the filarial N-glycoproteome by glycosite mapping in the human parasitic nematode Brugia malayi
Scientific Reports (2023)
-
C. elegans monitor energy status via the AMPK pathway to trigger innate immune responses against bacterial pathogens
Communications Biology (2022)
-
Engulfing cells promote neuronal regeneration and remove neuronal debris through distinct biochemical functions of CED-1
Nature Communications (2018)
-
Phosphatidylserine exposure mediated by ABC transporter activates the integrin signaling pathway promoting axon regeneration
Nature Communications (2018)
-
Transthyretin provides trophic support via megalin by promoting neurite outgrowth and neuroprotection in cerebral ischemia
Cell Death & Differentiation (2016)