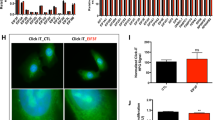
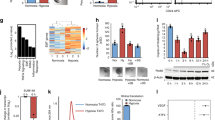
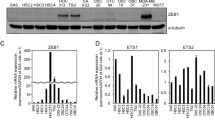
Abstract
Transforming growth factor-β (TGF-β) induces epithelial–mesenchymal transdifferentiation (EMT) accompanied by cellular differentiation and migration1,2,3,4,5. Despite extensive transcriptomic profiling, the identification of TGF-β-inducible, EMT-specific genes has met with limited success. Here we identify a post-transcriptional pathway by which TGF-β modulates the expression of EMT-specific proteins and of EMT itself. We show that heterogeneous nuclear ribonucleoprotein E1 (hnRNP E1) binds a structural, 33-nucleotide TGF-β-activated translation (BAT) element in the 3′ untranslated region of disabled-2 (Dab2) and interleukin-like EMT inducer (ILEI) transcripts, and represses their translation. TGF-β activation leads to phosphorylation at Ser 43 of hnRNP E1 by protein kinase Bβ/Akt2, inducing its release from the BAT element and translational activation of Dab2 and ILEI messenger RNAs. Modulation of hnRNP E1 expression or its post-translational modification alters the TGF-β-mediated reversal of translational silencing of the target transcripts and EMT. These results suggest the existence of a TGF-β-inducible post-transcriptional regulon that controls EMT during the development and metastatic progression of tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Massagué, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Bierie, B. & Moses, H. L. TGF-β and cancer. Cytokine Growth Factor Rev. 17, 29–40 (2006).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Zavadil, J. & Bottinger, E. P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nature Rev. Mol. Cell. Biol. 7, 131–142 (2006).
Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Thuault, S. et al. Transforming growth factor-β employs HMGA2 to elicit epithelial–mesenchymal transition. J. Cell Biol. 174, 175–183 (2006).
Oft, M. et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477 (1996).
Prunier, C. & Howe, P. H. Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT). J. Biol. Chem. 280, 17540–17548 (2005).
Waerner, T. et al. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell 10, 227–239 (2006).
Greinwald, J. H. Jr et al. Localization of a novel gene for nonsyndromic hearing loss (DFNB17) to chromosome region 7q31. Am. J. Med. Genet. 78, 107–113 (1998).
Zhu, Y. et al. Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics 80, 144–150 (2002).
Pradet-Balade, B., Boulme, F., Beug, H., Mullner, E. W. & Garcia-Sanz, J. A. Translation control: bridging the gap between genomics and proteomics? Trends Biochem. Sci. 26, 225–229 (2001).
Kang, Y. & Massagué, J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279 (2004).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Grillo, G., Licciulli, F., Liuni, S., Sbisa, E. & Pesole, G. PatSearch: a program for the detection of patterns and structural motifs in nucleotide sequences. Nucleic Acids Res. 31, 3608–3612 (2003).
Ostareck, D. H. et al. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89, 597–606 (1997).
Bakin, A. V., Tomlinson, A. K., Bhowmick, N. A., Moses, H. L. & Arteaga, C. L. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 (2000).
Kattla, J. J., Carew, R. M., Heljic, M., Godson, C. & Brazil, D. P. Protein kinase B/Akt activity is involved in renal TGF-β1-driven epithelial–mesenchymal transition in vitro and in vivo. Am. J. Physiol. Renal Physiol. 295, F215–F225 (2008).
Kato, M. et al. TGF-β activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nature Cell Biol. 11, 881–889 (2009).
Meng, Q. et al. Signaling-dependent and coordinated regulation of transcription, splicing, and translation resides in a single coregulator, PCBP1. Proc. Natl Acad. Sci. USA 104, 5866–5871 (2007).
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Brazil, D. P. & Hemmings, B. A. Ten years of protein kinase B signaling: a hard Akt to follow. Trends Biochem. Sci. 26, 657–664 (2001).
Kato, S., Ding, J. & Du, K. Differential activation of CREB by Akt1 and Akt2. Biochem. Biophys. Res. Commun. 354, 1061–1066 (2007).
Lamouille, S. & Derynck, R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 178, 437–451 (2007).
Irie, H. Y. et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial–mesenchymal transition. J. Cell Biol. 171, 1023–1034 (2005).
Keene, J. D. & Tenenbaum, S. A. Eukaryotic mRNPs may represent posttranscriptional operons. Mol. Cell 9, 1161–1167 (2002).
Nishinakamura, H. et al. An RNA-binding protein αCP-1 is involved in the STAT3-mediated suppression of NF-κB transcriptional activity. Int. Immunol. 19, 609–619 (2007).
Wildey, G. M., Patil, S. & Howe, P. H. Smad3 potentiates transforming growth factor-β (TGF-β)-induced apoptosis and expression of the BH3-only protein Bim in WEHI 231 B lymphocytes J. Biol. Chem. 278, 18069–18077 (2003).
Hocevar, B. A., Brown, T. L. & Howe, P. H. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18, 1345–1356 (1999).
Mazumder, B. & Fox, P. L. Delayed translational silencing of ceruloplasmin transcript in γ interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol. Cell. Biol. 19, 6898–6905 (1999).
Hampton, M. B., Zhivotovsky, B., Slater, A. F., Burgess, D. H. & Orrenius, S. Importance of the redox state of cytochrome c during caspase activation in cytosolic extracts. Biochem. J. 329, 95–99 (1998).
Ray, P. S. & Fox, P. L. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 26, 3360–3372 (2007).
Legagneux, V., Bouvet, P., Omilli, F., Chevalier, S. & Osborne, H. B. Identification of RNA-binding proteins specific to Xenopus Eg maternal mRNAs: association with the portion of Eg2 mRNA that promotes deadenylation in embryos. Development 116, 1193–1202 (1992).
Sampath, P. et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119, 195–208 (2004).
Qi, X. J., Wildey, G. M. & Howe, P. H. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J. Biol. Chem. 281, 813–823 (2006).
Acknowledgements
We thank Donna M. Driscoll, Barsanjit Mazumder and members of our laboratory for helpful discussions and critical insights. We value the assistance of Michael T. Kinter and Belinda Willard for liquid chromatography–mass spectrometry; of Judith A. Drazba and John Peterson for imaging; and of Michael Budiman for fast performance liquid chromatography. We thank Rakesh Kumar for the gift of the GST–hnRNP E1 construct, Takashi Kobayashi for the gifts of mouse pCMV14-hnRNP E1-Flag and psiRNA-hH1neo-mouse hnRNP E1, and Harold Moses for giving us the EpRas cell line. This work was supported by grants CA55536 and CA80095 from the National Cancer Institute to P.H.H. A.C. is supported by an American Heart Association (Ohio Valley Affiliate) Pre-doctoral Fellowship 075080B.
Author information
Authors and Affiliations
Contributions
P.H.H. directed the project. G.J. made the initial observation of uncoupled Dab2 mRNA and protein expression levels. G.S.H. performed the experiments in the EpRas cell line. P.S.R. contributed to the polysome profiling and PatSearch analyses. P.L.F. provided critical insights and expertise throughout. G.J., G.S.H. and A.C. made all the reagents. A.C. performed most of the experiments. P.H.H. and A.C. analysed the data and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3587 kb)
Rights and permissions
About this article
Cite this article
Chaudhury, A., Hussey, G., Ray, P. et al. TGF-β-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 12, 286–293 (2010). https://doi.org/10.1038/ncb2029
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2029
This article is cited by
-
Ubiquitination Process Mediates Prostate Cancer Development and Metastasis through Multiple Mechanisms
Cell Biochemistry and Biophysics (2024)
-
Disable 2, A Versatile Tissue Matrix Multifunctional Scaffold Protein with Multifaceted Signaling: Unveiling Role in Breast Cancer for Therapeutic Revolution
Cell Biochemistry and Biophysics (2024)
-
Disabled-2, a versatile tissue matrix multifunctional scaffold protein with multifaceted signaling: Unveiling its potential in the cancer battle
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
An mRNA processing pathway suppresses metastasis by governing translational control from the nucleus
Nature Cell Biology (2023)
-
PCBP1-mediated regulation of WNT signaling is critical for breast tumorigenesis
Cell Biology and Toxicology (2023)