Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is causally linked to several acquired immune deficiency syndrome-related malignancies, including Kaposi's sarcoma, primary effusion lymphoma (PEL) and a subset of multicentric Castleman's disease1. Control of viral lytic replication is essential for KSHV latency, evasion of the host immune system and induction of tumours1. Here, we show that deletion of a 14 microRNA (miRNA) cluster from the KSHV genome significantly enhances viral lytic replication as a result of reduced NF-κB activity. The miRNA cluster regulates the NF-κB pathway by reducing expression of IκBα protein, an inhibitor of NF-κB complexes. Computational and miRNA seed mutagenesis analyses were used to identify KSHV miR-K1, which directly regulates the IκBα protein level by targeting the 3′UTR of its transcript. Expression of miR-K1 is sufficient to rescue NF-κB activity and inhibit viral lytic replication, whereas inhibition of miR-K1 in KSHV-infected PEL cells has the opposite effect. Thus, KSHV encodes an miRNA to control viral replication by activating the NF-κB pathway. These results demonstrate an important role for KSHV miRNAs in regulating viral latency and lytic replication by manipulating the host survival pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ganem, D. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu. Rev. Pathol. 1, 273–296 (2006).
Cai, X. et al. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl Acad. Sci. USA 102, 5570–5575 (2005).
Pfeffer, S. et al. Identification of microRNAs of the herpesvirus family. Nature Methods 2, 269–276 (2005).
Samols, M. A., Hu, J., Skalsky, R. L. & Renne, R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79, 9301–9305 (2005).
Grundhoff, A., Sullivan, C. S. & Ganem, D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human γ-herpesviruses. RNA 12, 733–750 (2006).
O'Hara, A. J. et al. Pre-microRNA signatures delineate stages of endothelial cell transformation in Kaposi's sarcoma. PLoS Pathog. 5, e1000389 (2009).
Gottwein, E. et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature 450, 1096–1099 (2007).
Skalsky, R. L. et al. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. Virol. 81, 12836–12845 (2007).
Samols, M. A. et al. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 3, e65 (2007).
Ziegelbauer, J. M., Sullivan, C. S. & Ganem, D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nature Genet. 41, 130–134 (2009).
Nachmani, D., Stern-Ginossar, N., Sarid, R. & Mandelboim, O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe 5, 376–385 (2009).
Qin, Z., Kearney, P., Plaisance, K. & Parsons, C. H. Pivotal Advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J. Leukoc. Biol. 87, 25–34 (2010).
Zhou, F. C. et al. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76, 6185–6196 (2002).
Greene, W. et al. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 133, 69–127 (2007).
Murphy, E., Vanicek, J., Robins, H., Shenk, T. & Levine, A. J. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: implications for latency. Proc. Natl Acad. Sci. USA 105, 5453–5458 (2008).
Zhu, F. X., Cusano, T. & Yuan, Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73, 5556–5567 (1999).
Ye, F. C. et al. Kaposi's sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-κB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J. Virol. 82, 4235–4249 (2008).
Nelson, D. E. et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Ashall, L. et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science 324, 242–246 (2009).
Gao, S. J. et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nature Med. 2, 925–928 (1996).
Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005).
John, B. et al. Human MicroRNA targets. PLoS Biol. 2, e363 (2004).
Stern-Ginossar, N. et al. Host immune system gene targeting by a viral miRNA. Science 317, 376–381 (2007).
Grey, F., Meyers, H., White, E. A., Spector, D. H. & Nelson, J. A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication. PLoS Pathog. 3, e163 (2007).
Barth, S. et al. Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 36, 666–675 (2008).
Umbach, J. L. et al. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature (2008).
Ye, F. C. et al. Disruption of Kaposi's sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J. Virol. 78, 11121–11129 (2004).
Li, Q. H., Zhou, F. C., Ye, F. C. & Gao, S. J. Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology 379, 234–244 (2008).
Karin, M. NF-κB and cancer: mechanisms and targets. Mol. Carcinog. 45, 355–361 (2006).
Chaudhary, P. M., Jasmin, A., Eby, M. T. & Hood, L. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene 18, 5738–5746 (1999).
Matta, H. & Chaudhary, P. M. Activation of alternative NF-κB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 β-converting enzyme inhibitory protein (vFLIP). Proc. Natl Acad. Sci. USA 101, 9399–9404 (2004).
Mitchell, T. & Sugden, B. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69, 2968–2976 (1995).
Yoo, S. M., Zhou, F. C., Ye, F. C., Pan, H. Y. & Gao, S. J. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology 343, 47–64 (2005).
Chen, C. et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33, e179 (2005).
Xie, J. P., Ajibade, A. O., Ye, F. C., Kuhne, K. & Gao, S. J. Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology 371, 139–154 (2008).
Gottwein, E., Cai, X. & Cullen, B. R. A novel assay for viral microRNA function identifies a single nucleotide polymorphism that affects Drosha processing. J. Virol. 80, 5321–5326 (2006).
Gao, S. J. et al. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335, 233–241 (1996).
Wang, X. P. et al. Characterization of the promoter region of the viral interferon regulatory factor encoded by Kaposi's sarcoma-associated herpesvirus. Oncogene 20, 523–530 (2001).
Kurreck, J., Wyszko, E., Gillen, C. & Erdmann, V. A. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 30, 1911–1918 (2002).
Acknowledgements
We thank K. Izumi and A. Griffiths for their valuable suggestions. This work was supported by grants from the American Cancer Society (#RSG-04-195) and the National Institutes of Health (CA096512, CA124332, CA132637 and DE017333) to S.-J.G, and the National Science Foundation (CCF-0546345) to Y.F.H. C.-G.K. was supported by Konkuk University in 2008.
Author information
Authors and Affiliations
Contributions
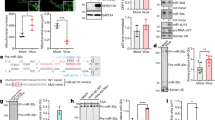
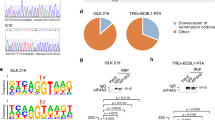
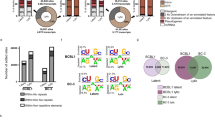
X.F.L. performed genetic, viral replication, NF-κB-related analysis and mRNA suppressor experiments (Figs 1, 2, 3, 4; Supplementary Information, Figs S1–S3, S5, S6 and S8); Z.Q.B. performed bioinformatics, mutagenesis of the miRNA target and part of the genetics and viral replication (Figs 2 and 3; Supplementary Information, Figs S1, S4, S7 and S8); X.P.X. conducted EMSA (Fig. 2); F.C.Y. contributed to viral genetics and northern blotting (Fig. 4; Supplementary Information, Fig. S1); C.G.K. contributed to target identification (Supplementary Information, Fig. S7); Y.F.H. contributed to bioinformatics (Fig. 3); S.J.G. directed, designed and analysed all the experiments and data; X.F.L. and S.J.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3537 kb)
Rights and permissions
About this article
Cite this article
Lei, X., Bai, Z., Ye, F. et al. Regulation of NF-κB inhibitor IκBα and viral replication by a KSHV microRNA. Nat Cell Biol 12, 193–199 (2010). https://doi.org/10.1038/ncb2019
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2019
This article is cited by
-
Pathogen regulatory RNA usage enables chronic infections, T-cell exhaustion and accelerated T-cell exhaustion
Molecular and Cellular Biochemistry (2023)
-
The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis
Infectious Agents and Cancer (2020)
-
Suppression of the SAP18/HDAC1 complex by targeting TRIM56 and Nanog is essential for oncogenic viral FLICE-inhibitory protein-induced acetylation of p65/RelA, NF-κB activation, and promotion of cell invasion and angiogenesis
Cell Death & Differentiation (2019)
-
Towards Better Understanding of KSHV Life Cycle: from Transcription and Posttranscriptional Regulations to Pathogenesis
Virologica Sinica (2019)