Abstract
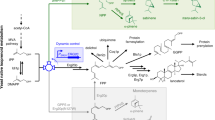
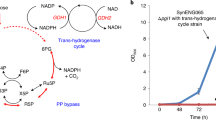
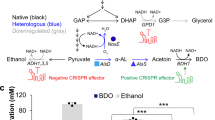
We report on the production of hydrocortisone, the major adrenal glucocorticoid of mammals and an important intermediate of steroidal drug synthesis, from a simple carbon source by recombinant Saccharomyces cerevisiae strains. An artificial and fully self-sufficient biosynthetic pathway involving 13 engineered genes was assembled and expressed in a single yeast strain. Endogenous sterol biosynthesis was rerouted to produce compatible sterols to serve as substrates for the heterologous part of the pathway. Biosynthesis involves eight mammalian proteins (mature forms of CYP11A1, adrenodoxin (ADX), and adrenodoxin reductase (ADR); mitochondrial forms of ADX and CYP11B1; 3β-HSD, CYP17A1, and CYP21A1). Optimization involved modulating the two mitochondrial systems and disrupting of unwanted side reactions associated with ATF2, GCY1, and YPR1 gene products. Hydrocortisone was the major steroid produced. This work demonstrates the feasibility of transfering a complex biosynthetic pathway from higher eukaryotes into microorganisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Woodward, R.B., Sondhermer, F., Taule, D., Hensler, K. & McLamore, W.H. The total synthesis of steroids. J. Am. Chem. Soc. 74, 4223 (1952).
Miller, W.L. Molecular biology of steroid hormone synthesis. Endocr. Rev. 9, 295–318 (1988).
Duport, C., Spagnoli, R., Degryse, E. & Pompon, D. Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat. Biotechnol. 16, 186–189 (1998).
Slater, S. et al. Metabolic engineering of Arabidopsis and Brassica for poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat. Biotechnol. 17, 1011–1016 (1999).
Dayem, L.C. et al. Metabolic engineering of a methylmalonyl-CoA mutase-epimerase pathway for complex polyketide biosynthesis in Escherichia coli. Biochemistry. 23, 5193–5201 (2002).
Dumas, B. et al. 11-β-hydroxylase activity in recombinant yeast mitochondria. In vivo conversion of 11-deoxycortisol to hydrocortisone. Eur. J. Biochem. 238, 495–504 (1996).
Lacour, T., Achstetter, T. & Dumas, B. Characterization of recombinant adrenodoxin reductase homologue (Arh1p) from yeast. Implication of in vitro cytochrome P450-11-β-monooxygenase system. J. Biol. Chem. 273, 23984–23992 (1998).
Cauet, G., Degryse, E., Ledoux, C., Spagnoli, R. & Achstetter, T. Pregnenolone esterification in Saccharomyces cerevisiae. A potential detoxification mechanism. Eur. J. Biochem. 261, 317–324 (1999).
Dumas, B., Cauet, G., Degryse, E., Spagnoli, R. & Achstetter, T. in Cytochrome P450. 8th International Conference (ed. Lechner, M.C.) 527–530 (John Libbey Eurotext, Montrouge, France 1994).
Shkumatov, V.M. et al. Biotransformation of steroids by a recombinant yeast strain expressing bovine cytochrome P450c17α. Biochemistry (Mosc.) 67, 456–467 (2002).
Zhang, Y., Dufort, I., Rheault, P. & Luu-The, V. Characterization of a human 20α-hydroxysteroid dehydrogenase. J. Mol. Endocrinol. 25, 221–228 (2000).
Angermayr, M. & Bandlow, W. The general regulatory factor Reb1p controls basal, but not Gal4p- mediated, transcription of the GCY1 gene in yeast. Mol. Gen. Genet. 256, 682–689 (1997).
Manzella, L., Barros, M.H. & Nobrega, F.G. ARH1 of Saccharomyces cerevisiae: a new essential gene that codes for a protein homologous to the human adrenodoxin reductase. Yeast 14, 839–846 (1998).
Metherall, J.E., Waugh, K. & Li, H. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J. Biol. Chem. 271, 2627–2633 (1996).
Sakaki, T. et al. Progesterone metabolism in recombinant yeast simultaneously expressing bovine cytochromes P450c17 (CYP17A1) and P450c21 (CYP21B1) and yeast NADPH-P450 oxidoreductase. Pharmacogenetics 1, 86–93 (1991).
Degryse, E., Cauet, G., Spagnoli, R. & Achstetter, T. Pregnenolone metabolized to 17α-hydroxyprogesterone in yeast: biochemical analysis of a metabolic pathway. J. Steroids Biochem. Mol. Biol. 71, 239–246 (1999).
Barros, M.H., Nobrega, F.G. & Tzagoloff, A. Mitochondrial ferredoxin is required for heme A synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 277, 9997–10002 (2002).
Milgrom, E. in Hormones (eds. Beaulieu, E.E. & Kelly, P.A.) 387–437 (Herman Publishers in Arts and Science, New York, 1990).
Degryse, E. In vivo intermolecular recombination in Escherichia coli: application to plasmid constructions. Gene 170, 45–50 (1996).
Burk, D., Dawson, D. & Stearns, T. Methods in Yeast Genetics (Cold Spring Harbor Laboratory Press, Plainview, NY, 2000).
Magdolen, V., Oechsner, U., Trommler, P. & Bandlow, W. Transcriptional control by galactose of a yeast gene encoding a protein homologous to mammalian aldo-keto reductases. Gene 90, 105–114 (1990).
Thierry, A., Fairhead, C. & Dujon, B. The complete sequence of the 8.2 kb segment left of MAT on chromosome III reveals five ORFs, including a gene for a yeast ribokinase. Yeast 6, 521–534 (1990).
Struhl, K., Stinchcomb, D.T., Scherer, S. & Davis, R.W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 76, 1035–1039 (1979).
Wach, A., Brachat, A., Pohlmann, R. & Philippsen, P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 (1994).
Kappeli, O., Arreguin, M. & Rieger, M. The respirative breakdown of glucose by Saccharomyces cerevisiae: an assessment of a physiological state. J. Gen. Microbiol. 131, 1411–1416 (1985).
Yanisch-Perron, C., Vieira, J. & Messing, J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33, 103–119 (1985).
Lathe, R., Vilotte, J.L. & Clark, A.J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene 57, 193–201 (1987).
Bonneaud, N. et al. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7, 609–615 (1991).
Lacour, T. & Dumas, B. A gene encoding a yeast equivalent of mammalian NADPH-adrenodoxin oxidoreductases. Gene 174, 289–292 (1996).
Degryse, E., Dumas, B., Dietrich, M., Laruelle, L. & Achstetter, T. In vivo cloning by homologous recombination in yeast using a two-plasmid-based system. Yeast 11, 629–640 (1995).
Acknowledgements
This work was funded by Aventis Pharma France. We are in debt to Jacques Raynaud and the late Jean Pierre Lecocq for starting this innovative project, and to Udo Hedtmann for his continuous support. We also thank Francis Karst and Mike Waterman for providing us with their advice during the many years of this exciting adventure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Szczebara, F., Chandelier, C., Villeret, C. et al. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol 21, 143–149 (2003). https://doi.org/10.1038/nbt775
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt775
This article is cited by
-
Synthesis of mono Cytochrome P450 in a modified CHO-CPR cell-free protein production platform
Scientific Reports (2024)
-
Light-driven progesterone production by InP–(M. neoaurum) biohybrid system
Bioresources and Bioprocessing (2022)
-
Unlocking the access to oxidized coenzyme A via a single-step green membrane-based purification
Scientific Reports (2022)
-
Increased biosynthesis of acetyl-CoA in the yeast Saccharomyces cerevisiae by overexpression of a deregulated pantothenate kinase gene and engineering of the coenzyme A biosynthetic pathway
Applied Microbiology and Biotechnology (2021)
-
Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae
Applied Microbiology and Biotechnology (2021)