Abstract
Cancer cells hijack and remodel existing metabolic pathways for their benefit. Argininosuccinate synthase (ASS1) is a urea cycle enzyme that is essential in the conversion of nitrogen from ammonia and aspartate to urea. A decrease in nitrogen flux through ASS1 in the liver causes the urea cycle disorder citrullinaemia1. In contrast to the well-studied consequences of loss of ASS1 activity on ureagenesis, the purpose of its somatic silencing in multiple cancers is largely unknown2. Here we show that decreased activity of ASS1 in cancers supports proliferation by facilitating pyrimidine synthesis via CAD (carbamoyl-phosphate synthase 2, aspartate transcarbamylase, and dihydroorotase complex) activation. Our studies were initiated by delineating the consequences of loss of ASS1 activity in humans with two types of citrullinaemia. We find that in citrullinaemia type I (CTLN I), which is caused by deficiency of ASS1, there is increased pyrimidine synthesis and proliferation compared with citrullinaemia type II (CTLN II), in which there is decreased substrate availability for ASS1 caused by deficiency of the aspartate transporter citrin. Building on these results, we demonstrate that ASS1 deficiency in cancer increases cytosolic aspartate levels, which increases CAD activation by upregulating its substrate availability and by increasing its phosphorylation by S6K1 through the mammalian target of rapamycin (mTOR) pathway. Decreasing CAD activity by blocking citrin, the mTOR signalling, or pyrimidine synthesis decreases proliferation and thus may serve as a therapeutic strategy in multiple cancers where ASS1 is downregulated. Our results demonstrate that ASS1 downregulation is a novel mechanism supporting cancerous proliferation, and they provide a metabolic link between the urea cycle enzymes and pyrimidine synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dimmock, D. et al. Citrin deficiency, a perplexing global disorder. Mol. Genet. Metab. 96, 44–49 (2009)
Delage, B. et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 126, 2762–2772 (2010)
Long, Y. et al. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol. Cancer Ther. 12, 2581–2590 (2013)
Morris, S. M., Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br. J. Pharmacol. 157, 922–930 (2009)
Wheatley, D. N. Controlling cancer by restricting arginine availability–arginine-catabolizing enzymes as anticancer agents. Anticancer Drugs 15, 825–833 (2004)
Syed, N. et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 4, e458 (2013)
Duarte, N. C. et al. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl Acad. Sci. USA 104, 1777–1782 (2007)
Kobayashi, K., Saheki, T. & Song, Y. Z. Citrin deficiency. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1181/ (2005)
Marion, V. et al. Hepatic adaptation compensates inactivation of intestinal arginine biosynthesis in suckling mice. PLoS One 8, e67021 (2013)
Yizhak, K. et al. Phenotype-based cell-specific metabolic modeling reveals metabolic liabilities of cancer. eLife 3, e03641 (2014)
Kuijjer, M. L. et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 13, 245 (2013)
Kabbarah, O. et al. Integrative genome comparison of primary and metastatic melanomas. PLoS One 5, e10770 (2010)
del Arco, A. et al. Expression of the aspartate/glutamate mitochondrial carriers aralar1 and citrin during development and in adult rat tissues. Eur. J. Biochem. 269, 3313–3320 (2002)
Tan, G. S. et al. Novel proteomic biomarker panel for prediction of aggressive metastatic hepatocellular carcinoma relapse in surgically resectable patients. J. Proteome Res. 13, 4833–4846 (2014)
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013)
Folger, O. et al. Predicting selective drug targets in cancer through metabolic networks. Mol. Syst. Biol. 7, 501 (2011)
Varma, A. P. & Palsson, B. O. Metabolic flux balancing: basic concepts, scientific and practical use. Nature Biotechnol. 12, 994–998 (1994)
Bordel, S., Agren, R. & Nielsen, J. Sampling the solution space in genome-scale metabolic networks reveals transcriptional regulation in key enzymes. PLOS Comput. Biol. 6, e1000859 (2010)
Lee, J. K. et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc. Natl Acad. Sci. USA 104, 13086–13091 (2007)
Itzkovitz, S. et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nature Cell Biol. 14, 106–114 (2012)
Gu, G. J. et al. Protein tag-mediated conjugation of oligonucleotides to recombinant affinity binders for proximity ligation. New Biotechnol. 30, 144–152 (2013)
Hao, G., Xie, L. & Gross, S. S. Argininosuccinate synthetase is reversibly inactivated by S-nitrosylation in vitro and in vivo. J. Biol. Chem. 279, 36192–36200 (2004)
Bjorklund, N. L., Sadagoparamanujam, V. M. & Taglialatela, G. Selective, quantitative measurement of releasable synaptic zinc in human autopsy hippocampal brain tissue from Alzheimer’s disease patients. J. Neurosci. Methods 203, 146–151 (2012)
Robitaille, A. M. et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323 (2013)
Zhang, Y. et al. Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J. Biol. Chem. 276, 20913–20923 (2001)
Pinthus, J. H. et al. WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res. 60, 6563–6567 (2000)
Acknowledgements
We thank A. Gross and B. Lee for discussions. We acknowledge and thank the Weizmann Institute for providing financial and infrastructural support and the Baylor College of Medicine Biochemical Laboratory. We appreciate the statistical analysis by R. Rotkopf and the technical contributions of A. Tishbee, T. Kaufman, D. Laufer and I. Rogachev. A.E. is the incumbent of the Leah Omenn Career Development Chair and is supported by research grants from the European Research Program (CIG618113, ERC614204), the Israel Science Foundation (1343/13; 1952/13) and a Minerva grant award (711730). A.E. received additional support from the Adelis Foundation, the Henry S. and Anne S. Reich Research Fund, the Dukler Fund for Cancer Research, the Paul Sparr Foundation, the Saul and Theresa Esman Foundation, from Joseph Piko Baruch and from the estate of Fannie Sherr. L.A. was supported by a postdoctoral fellowship from Teva. The research of K.Y. and E.R. has been supported by grants from the Israeli Science Foundation (E.R.), the Israeli Cancer Research Fund (E.R.), the Israeli Center of Excellence (I-CORE) Program of the Planning and Budgeting Committee and Israel Science Foundation Grant No. 41/11 (E.R.). A.Sa. is supported by the Israel Cancer Research Foundation and S.N.S.C. is supported by a Baylor College of Medicine Intellectual and Developmental Disabilities Research Center Grant (number 1 U54 HD083092) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, and by the Doris Duke Charitable Foundation (DDCF 2013095). I.U. was supported by a grant from the Rising Tide Foundation and by a research grant from The Abramson Family Center for Young Scientists.
Author information
Authors and Affiliations
Contributions
S.R. performed most of the experiments described in the manuscript; L.A. set up the system that allowed us to test our hypothesis; K.Y and E.R designed the modelling analysis. K.Y. from the laboratory of E.R. performed the modelling analysis; A.Sa. performed in vitro studies with the patients’ cells and helped establish the in vivo models; A.Si. executed the metabolic analysis by gas chromatography–mass spectrometry (GC–MS); S.A. helped with the si-citrin experiments; N.S. performed the in vivo experiments; Q.S. analysed levels of orotic acid in the patients’ urine; A.B. performed the pyrimidine analysis by liquid chromatography–mass spectrometry (LC–MS); D.H., S.K. and D.D. provided data and primary cell lines from patients with CTLN II; S.I. and S.A. performed the fluorescence in situ hybridization (FISH) experiments of the mouse intestine; I.U. analysed the TCGA database; S.C.S.N. provided primary cells of patients with CTLN I as well as help writing the manuscript; A.E. was the leading principal investigator who initiated and directed the study and co-wrote the paper with inputs from all authors. K.Y., A.Si., S.H. and A.Sa. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
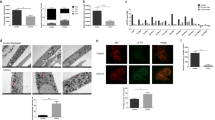
Extended Data Figure 1 ASS1 deficiency correlates with aspartate utilization by CAD in cancerous and non-cancerous cells.
a, Schematic flux tracing of the α-labelled nitrogen of glutamine ([15N]α-glutamine) to nucleic acid synthesis via aspartate. b, The ratio between M + 1-labelled and total uracil in fibroblasts is similar between patients with citrullinaemia patients and control subjects (n ≥ 3). Error bars are standard error. c, Labelled levels of M + 1 aspartate (left) and M + 1 uracil (right) synthesized from [15N]α-glutamine are higher in fibroblasts from CTLN I than in fibroblasts from controls and patients with CTLN II (n ≥ 3). d, TCGA analysis of tumour–normal paired tissues for gene expression comparison shows the expression levels of ASL and ASS1 in different cancers. e, Plot generated from the modelling data for the production capacity of metabolites after ASS1 inactivation in each of the NCI-60 cell lines as well as in the generic model. The reddish bars represent the ranking of nucleic acids while the blueish bars represent the ranking of all other metabolites. f, Correlation analysis of NCI-60 cell lines shows a significant inverse correlation between ASS1 and CAD expression levels. g, Osteosarcoma (upper) and melanoma (lower) microarray data was obtained from the NCBI EO database (accession numbers GSE33383 and GSE46517, respectively). Raw expression levels were plotted and significance was computed using a t-test on log2-transformed expression levels. The number of patients for each subtype is shown in parenthesis on the left. h, Western blot for CAD and ASS1 shows higher expression levels of CAD in the MNNG/HOS human osteosarcoma cell line, which has a low expression level of ASS1 compared with U2OS, which has higher expression levels of ASS1; p97 is shown as loading control.
Extended Data Figure 2 ASS1 inactivation in melanoma correlates with increased proliferation.
a, An immunoblot showing different expression levels of ASS1 and CAD in two different cancer cell lines of melanoma. b, Melanoma cells with ASS1 downregulation have a significant increase in pyrimidine levels as measured by LC–MS (n ≥ 3). c, Melanoma cells with ASS1 downregulation have a significant increase in total uracil (n = 4). d, Melanoma cells with ASS1 downregulation have a significant increase in proliferation as measured by MTT assay (n = 2). e, Immunoblots of melanoma cells for ASS1 levels after transduction with either ASS1 over expression construct or with shASS1. f, Proliferation assays showing a significant decrease in proliferation after ASS1 overexpression in melanoma using MTT (n = 3). g, Crystal violet quantification for melanoma cells after transduction with shASS1 demonstrating increase in proliferation (n = 3). h, LC–MS measurements of pyrimidine levels showing a significant increase after the use of shASS1 in melanoma cells (n ≥ 3). i, Left: total uracil levels are decreased significantly in melanoma cells with ASS1 overexpression and increased in melanoma cells with shASS1 (right) (n ≥ 2). j, Significant increase in proliferation of melanoma cells by dNTPs after ASS1 overexpression (n = 3). All error bars are standard errors.
Extended Data Figure 3 Downregulation of ASS1 levels increases pyrimidine synthesis.
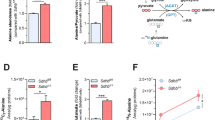
a, Osteosarcoma cells were transduced with two different shASS1 vectors: shASS742 and shASS745. Both clones decreased ASS1 levels efficiently to approximately 20% expression (left), resulting in a significant increase in uracil M + 1 levels (b) and in proliferation (n ≥ 3) (c). d, RNA levels measured in U2OS at 24 h intervals show increased levels of RNA in U2OS infected with shASS1 compared with the empty vector. e, Uracil M + 1 levels increase more in U2OS infected with shASS1 compared with the empty vector during 38 h of measurements. f, The levels of total and labelled M + 1 alanine synthesis from [15N]α-glutamine do not change significantly after ASS1 downregulation (n = 3). g, Tumours with shASS1 had higher levels of M + 1 aspartate (left) and M + 1 uracil (right) synthesized from [15N]α-glutamine, compared with tumours expressing the empty vector (n = 15). All error bars are standard errors.
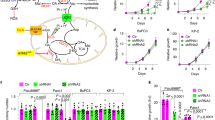
Extended Data Figure 4 Cancers with ASS1 downregulation are addicted to aspartate.
a, b, Analysis of the TCGA database of matched tumour–normal pairs showing no significant difference in the expression level of citrin in tissues with a high baseline expression of citrin (a) and significant elevation in tumours in which the normal tissue has low basal expression of citrin (b) (*P < 0.001). c, Immunoblot showing the expression level of citrin in osteosarcoma cells after si-citrin. d, Labelled and unlabelled aspartate (top) and uracil (bottom) are elevated significantly in cancers with ASS1 downregulation and are comparable to control in cells with both ASS1 and citrin downregulation (n ≥ 3). Error bars are standard errors. e, Kaplan–Meier survival analysis for two different cancer types (BRCA, breast cancer (top); LUSC, lung squamous cell carcinoma (bottom)), showing poor survival trend for cancers with low ASS1 and high citrin. For each cancer type, the Kaplan–Meier plot indicates the survival rates of four groups of patients: (1) ASS1 low expression and citrin high expression; (2) ASS1 low expression; (3) citrin high expression; (4) none of these. We analysed the cancer types for which there were sufficient survival data. f, Quantification graph of a western blot showing decreased CAD and S6K phosphorylation after treatment of U2OS with si-citrin. Error bars are standard errors. g, Proximity ligation assay showing increased proximity between CAD and citrin after ASS1 knockdown in U2OS cells (top, red dots). The left and middle pictures show the proximity between ASS1 and CAD to citrin in U2OS infected with empty vector, whereas the right picture shows the proximity between CAD and citrin after infection of U2OS with shASS1. Bottom: quantification of proximity ligation assays performed on U2OS infected with either empty vector (EV) or with shASS1 using antibodies for citrin, ASS1 and CAD. The pictures were quantified using ImageJ.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-2 and Supplementary Table 1. (PDF 399 kb)
Rights and permissions
About this article
Cite this article
Rabinovich, S., Adler, L., Yizhak, K. et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 527, 379–383 (2015). https://doi.org/10.1038/nature15529
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15529
This article is cited by
-
Metabolic reprogramming in colorectal cancer: regulatory networks and therapy
Cell & Bioscience (2023)
-
Gain-of-function mutant p53 together with ERG proto-oncogene drive prostate cancer by beta-catenin activation and pyrimidine synthesis
Nature Communications (2023)
-
CHIP promotes CAD ubiquitination and degradation to suppress the proliferation and colony formation of glioblastoma cells
Cellular Oncology (2023)
-
A role for macrophages under cytokine control in mediating resistance to ADI-PEG20 (pegargiminase) in ASS1-deficient mesothelioma
Pharmacological Reports (2023)
-
p53 inhibits the Urea cycle and represses polyamine biosynthesis in glioma cell lines
Metabolic Brain Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.