Abstract
Speciation generally involves a three-step process—range expansion, range fragmentation and the development of reproductive isolation between spatially separated populations1,2. Speciation relies on cycling through these three steps and each may limit the rate at which new species form1,3. We estimate phylogenetic relationships among all Himalayan songbirds to ask whether the development of reproductive isolation and ecological competition, both factors that limit range expansions4, set an ultimate limit on speciation. Based on a phylogeny for all 358 species distributed along the eastern elevational gradient, here we show that body size and shape differences evolved early in the radiation, with the elevational band occupied by a species evolving later. These results are consistent with competition for niche space limiting species accumulation5. Even the elevation dimension seems to be approaching ecological saturation, because the closest relatives both inside the assemblage and elsewhere in the Himalayas are on average separated by more than five million years, which is longer than it generally takes for reproductive isolation to be completed2,3,6; also, elevational distributions are well explained by resource availability, notably the abundance of arthropods, and not by differences in diversification rates in different elevational zones. Our results imply that speciation rate is ultimately set by niche filling (that is, ecological competition for resources), rather than by the rate of acquisition of reproductive isolation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mayr, E. & Diamond, J. M. The Birds of Northern Melanesia: Speciation, Ecology, and Biogeography (Oxford Univ. Press, 2001)
Price, T. Speciation in Birds (Roberts, 2008)
Weir, J. T. & Price, T. D. Limits to speciation inferred from times to secondary sympatry and ages of hybridizing species along a latitudinal gradient. Am. Nat. 177, 462–469 (2011)
Goldberg, E. E. & Lande, R. Ecological and reproductive character displacement on an environmental gradient. Evolution 60, 1344–1357 (2006)
Harvey, P. H. & Rambaut, A. Comparative analyses for adaptive radiations. Phil. Trans. R. Soc. Lond. B 355, 1599–1605 (2000)
Price, T. D. & Bouvier, M. M. The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089 (2002)
Rosenzweig, M. L. Species Diversity in Space and Time (Cambridge Univ. Press, 1995)
Endler, J. A. Geographic Variation, Speciation, and Clines (Princeton Univ. Press, 1977)
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000)
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, 2000)
Grant, P. R. & Grant, B. R. How and Why Species Multiply (Princeton Univ. Press, 2008)
Yoder, J. B. et al. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596 (2010)
Johansson, U. S. et al. Build-up of the Himalayan avifauna through immigration: a biogeographical analysis of the Phylloscopus and Seicercus warblers. Evolution 61, 324–333 (2007)
Richman, A. D. & Price, T. Evolution of ecological differences in the Old World leaf warblers. Nature 355, 817–821 (1992)
Ghosh-Harihar, M. & Price, T. D. A test for community saturation along the Himalayan bird diversity gradient, based on within-species geographical variation. J. Anim. Ecol. 628–638 (2014)
Jønsson, K. A., Fabre, P. H., Ricklefs, R. E. & Fjeldså, J. A major global radiation of corvoid birds originated in the proto-Papuan archipelago. Proc. Natl Acad. Sci. USA 108, 2328–2333 (2011)
Päckert, M. et al. Horizontal and elevational phylogeographic patterns of Himalayan and Southeast Asian forest passerines (Aves: Passeriformes). J. Biogeogr. 39, 556–573 (2012)
Kennedy, J. D. et al. Ecological limits on diversification of the Himalayan core Corvoidea. Evolution 66, 2599–2613 (2012)
Price, T. D. The roles of time and ecology in the continental radiation of the Old World leaf warblers (Phylloscopus and Seicercus) . Phil. Trans. R. Soc. Lond. B 365, 1749–1762 (2010)
Wiens, J. J., Parra-Olea, G., Garcia-Paris, M. & Wake, D. B. Phylogenetic history underlies elevational biodiversity patterns in tropical salamanders. Proc. R. Soc. Lond. B 274, 919–928 (2007)
Price, T. D. et al. Determinants of northerly range limits along the Himalayan bird diversity gradient. Am. Nat. 178, S97–S108 (2011)
MacArthur, R. H. Patterns of species diversity. Biol. Rev. Camb. Philos. Soc. 40, 510–533 (1965)
Ricklefs, R. E. Species richness and morphological diversity of passerine birds. Proc. Natl Acad. Sci. USA 109, 14482–14487 (2012)
Rosenzweig, M. L. & Abramsky, Z. in Species Diversity in Ecological Communities (eds Ricklefs, R. E. & Schluter, D. ) 52–65 (Univ. Chicago, 1993)
Rabosky, D. L. & Matute, D. R. Macroevolutionary speciation rates are decoupled from the evolution of intrinsic reproductive isolation in Drosophila and birds. Proc. Natl Acad. Sci. USA 110, 15354–15359 (2013)
Cornell, H. V. Is regional species diversity bounded or unbounded? Biol. Rev. Camb. Phil. Soc. 88, 140–165 (2013)
Ricklefs, R. E. & Bermingham, E. The concept of the taxon cycle in biogeography. Glob. Ecol. Biogeogr. 11, 353–361 (2002)
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007)
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E. & Challenger, W. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131 (2008)
Helmus, M. R., Bland, T. J., Williams, C. K. & Ives, A. R. Phylogenetic measures of biodiversity. Am. Nat. 169, E68–E83 (2007)
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012)
Price, T. Correlated evolution and independent contrasts. Phil. Trans. R. Soc. Lond. B 352, 519–529 (1997)
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005)
Losos, J. B. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639 (2010)
Mayr, E. Systematics and the Origin of Species from the Viewpoint of a Zoologist (Columbia Univ. Press, 1942)
Nosil, P. Ecological Speciation (Oxford Univ. Press, 2012)
Price, T., Zee, J., Jamdar, K. & Jamdar, N. Bird species diversity along the Himalayas: a comparison of Himachal Pradesh with Kashmir. J. Bombay Nat. Hist. Soc. 100, 394–409 (2003)
Harvey, P. H., May, R. M. & Nee, S. Phylogenies without fossils. Evolution 48, 523–529 (1994)
Phillimore, A. B. & Price, T. D. Density-dependent cladogenesis in birds. PLoS Biol. 6, 483–489 (2008)
Phillimore, A. B. & Price, T. D. in Speciation and Patterns of Diversity (eds Butlin, R. K., Bridle, J. & Schluter, D. ) 240–256 (Cambridge Univ. Press, 2009)
Weir, J. T. Divergent patterns of species accumulation in lowland and highland neotropical birds. Evolution 60, 842–855 (2006)
Etienne, R. S. & Rosindell, J. Prolonging the past counteracts the pull of the present: protracted speciation can explain observed slowdowns in diversification. Syst. Biol. 61, 204–213 (2012)
Pybus, O. G. & Harvey, P. H. Testing macro-evolutionary models using incomplete molecular phylogenies. Proc. R. Soc. Lond. B 267, 2267–2272 (2000)
McPeek, M. A. The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270–E284 (2008)
Rabosky, D. L. Diversity-dependence, ecological speciation, and the role of competition in macroevolution. Annu. Rev. Ecol. Evol. Syst. 44, 481–502 (2013)
Ricklefs, R. E. Community diversity: relative roles of local and regional processes. Science 235, 167–171 (1987)
Coyne, J. A. & Price, T. D. Little evidence for sympatric speciation in island birds. Evolution 54, 2166–2171 (2000)
Gill, F. B. Ecology and evolution of sympatric Mascarene white-eyes, Zosterops borbonica and Zosterops olivacea . Auk 88, 35–60 (1971)
Badgley, C. et al. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc. Natl Acad. Sci. USA 105, 12145–12149 (2008)
Revell, L. J. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 (2009)
Beaulieu, J. M., Jhwueng, D. C., Boettiger, C. & O'Meara, B. C. Modeling stabilizing selection: expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383 (2012)
Butler, M. A. & King, A. A. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 164, 683–695 (2004)
Ricklefs, R. E. Cladogenesis and morphological diversification in passerine birds. Nature 430, 338–341 (2004)
Blomberg, S. P., Garland, T. & Ives, A. R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 (2003)
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010)
Mahler, D. L., Revell, L. J., Glor, R. E. & Losos, J. B. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean Anoles. Evolution 64, 2731–2745 (2010)
Rasmussen, P. C. & Anderton, J. C. Birds of South Asia: the Ripley Guide (Lynx, 2005)
Weir, J. T. & Schluter, D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (2007)
Dong, F., Li, S. H. & Yang, X. J. Molecular systematics and diversification of the Asian scimitar babblers (Timaliidae, Aves) based on mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 57, 1268–1275 (2010)
Groth, J. G. & Barrowclough, G. F. Basal divergences in birds and the phylogenetic utility of the nuclear RAG-1 gene. Mol. Phylogenet. Evol. 12, 115–123 (1999)
Nylander, J. A. A., Ronquist, F., Huelsenbeck, J. P. & Nieves-Aldrey, J. L. Bayesian phylogenetic analysis of combined data. Syst. Biol. 53, 47–67 (2004)
Swofford, D. L. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) Version 4 (Sinauer, 2003)
Ayres, D. L. et al. BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Syst. Biol. 61, 170–173 (2012)
Linder, H. P., Hardy, C. R. & Rutschmann, F. Taxon sampling effects in molecular clock dating: an example from the African Restionaceae. Mol. Phylogenet. Evol. 35, 569–582 (2005)
Rambaut, A. & Drummond, A. J. Tracer v1.4. http://beast.bio.ed.ac.uk/ (2007)
Weir, J. T. & Schluter, D. Calibrating the avian molecular clock. Mol. Ecol. 17, 2321–2328 (2008)
Hackett, S. J. et al. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 (2008)
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004)
Mayr, G. The age of the crown group of passerine birds and its evolutionary significance—molecular calibrations versus the fossil record. Syst. Biodivers. 11, 7–13 (2013)
Hansen, J. et al. Target atmospheric CO2: where should humanity aim? Open Atmos. Sci. J. 2, 217–231 (2008)
Kraatz, B. P. & Geisler, J. H. Eocene-Oligocene transition in Central Asia and its effects on mammalian evolution. Geology 38, 111–114 (2010)
Helmus, M. R. & Ives, A. R. Phylogenetic diversity-area curves. Ecology 93, S31–S43 (2012)
Laliberté, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010)
Laliberté, E. & Shipley, B. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1. 0-11, http://cran.r-project.org/web/packages/FD/index.html (2011)
Acharya, B. K., Sanders, N. J., Vijayan, L. & Chettri, B. Elevational gradients in bird diversity in the eastern Himalaya: an evaluation of distribution patterns and their underlying mechanisms. PLoS ONE 6, e29097 (2011)
Gross, S. & Price, T. Determinants of the northern and southern range limits of a warbler. J. Biogeogr. 27, 869–878 (2000)
Ghosh-Harihar, M. Distribution and abundance of foliage-arthropods across elevational gradients in the east and west Himalayas. Ecol. Res. 28, 125–130 (2013)
Pebesma, E. J. & Bivand, R. S. Classes and methods for spatial data in R. R News 5 http://cran.r-project.org/doc/Rnews/ (2005)
Bivand, R. S., Pebesma, E. J. & Gomez-Rubio, V. Applied Spatial Data Analysis with R. (Springer, 2008)
Hijmans, R. J. & van Etten, J. Raster: geographic analysis and modeling with raster data. R package version 1. 9-92, http://CRAN.R-project.org (2011)
Schnute, J. T., Boers, N. M. & Haigh, R. PBS Mapping 2: user’s guide. Can. Tech. Rep. Fish. Aquat. Sci. 2549, 126 (2004)
Rahbek, C. The elevational gradient of species richness—a uniform pattern. Ecography 18, 200–205 (1995)
McCain, C. M. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 18, 346–360 (2009)
Terborgh, J. Bird species diversity on an Andean elevational gradient. Ecology 58, 1007–1019 (1977)
Sanders, N. J. & Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 35, 1–3 (2012)
Grytnes, J. A. & Vetaas, O. R. Species richness and altitude: a comparison between null models and interpolated plant species richness along the Himalayan altitudinal gradient, Nepal. Am. Nat. 159, 294–304 (2002)
Hawkins, B. A., Diniz, J. A. F. & Weis, A. E. The mid-domain effect and diversity gradients: is there anything to learn? Am. Nat. 166, E140–E143 (2005)
Zapata, F. A., Gaston, K. J. & Chown, S. L. The mid-domain effect revisited. Am. Nat. 166, E144–E148 (2005)
Colwell, R. K., Rahbek, C. & Gotelli, N. J. The mid-domain effect: there’s a baby in the bathwater. Am. Nat. 166, E149–E154 (2005)
McCain, C. M. The mid-domain effect applied to elevational gradients: species richness of small mammals in Costa Rica. J. Biogeogr. 31, 19–31 (2004)
Grytnes, J. A., Heegaard, E. & Romdal, T. S. Can the mass effect explain the mid-altitudinal peak in vascular plant species richness? Basic Appl. Ecol. 9, 373–382 (2008)
White, E. P. & Hurlbert, A. H. The combined influence of the local environment and regional enrichment on bird species richness. Am. Nat. 175, E35–E43 (2010)
Terborgh, J. W. & Faaborg, J. Saturation of bird communities in the West Indies. Am. Nat. 116, 178–195 (1980)
Acknowledgements
We thank the government of India and the Chief Wildlife Wardens of the six Indian Himalayan states for permits. We also thank S. Dalvi, K. Jamdar, N. Jamdar, E. Scordato and D. Wheatcroft for help in the field, U. Borthakur and V. Mathur in the laboratory and E. Goldberg, R. Hudson, J. Kennedy, M. McPeek, A. Phillimore, D. Schluter, T. Tyrberg and J. Weir for advice. Tissue and toe pads for this project were provided by J. Cracraft (American Museum of Natural History), N. Rice (Academy of Natural Sciences, Philadelphia), M. Adams (The Natural History Museum, Tring), J. Dumbacher and M. Flannery (California Academy of Sciences), J. Bates and D. Willard (Field Museum of Natural History, Chicago), Herman Mays (Cincinnatti Museum); M. Wink (Institut für Pharmazie und Molekulare Biotechnologie, Heidelberg); R. Brumfeld and D. Dittmann (Lousiana State Museum of Zoology); S. Edwards (Museum of Natural History, Harvard); G. Frisk (Swedish Museum of Natural History, Stockholm); J. Dean and J. Rappole (National Museum of Natural History Smithsonian); S. Birks (Burke Museum, University of Washington); the Zoologisches Forschungmuseum Alexander Koenig, Bonn; J. Bolding Kristensen and J. Fjeldså (Zoological Museum, Copenhagen); the Zoologische Staatssammlung München, Munich; and K. Zyskowski (Yale University). This work was supported in part by grants from the US NSF and the National Geographic Society (TDP), the Jornvall Foundation, a Chinese Academy of Sciences Visiting Professorship (to P.A.), the Swedish Research Council (grants to U.O. and P.A.), the Wenner-Gren Foundation (a grant to U.S.J.), the Feldbausch Foundation of Mainz University (a grant to J.M.) and the German DFG (grants to B.H. and D.T.T., grant number Ti 679/1-1). We thank D. Tautz for making laboratory facilities available in Germany.
Author information
Authors and Affiliations
Contributions
Study design and fieldwork: T.D.P. and D.M. Logistics: D.M. and P.S. Field collections: D.T.T., D.M.H., U.S.J., P.A., U.O., F.I., J.M., D.M. and T.D.P. Sequencing: D.M.H., U.S.J., D.T.T., U.O., P.A. and B.H. Arthropod censuses: M.G.-H. and T.D.P. Phylogeny construction: D.M.H., with early input from D.T.T. and P.A. Museum measurements: C.D.B., D.M., T.D.P. and U.S.J. Analysis and manuscript preparation: T.D.P., with input from B.H., D.M., D.M.H., D.T.T., P.A., U.S.J. and U.O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 The speciation cycle.
A species distributed across space becomes fragmented as a result of either vicariance (illustrated) or dispersal. After barrier formation, reproductive isolation develops. For the cycle to continue at least one of the species must expand into the other’s range, which requires reproductive isolation, and generally also ecological compatibility.
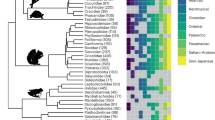
Extended Data Figure 2 Close relatives of a single Himalayan species, the Oriental white-eye, Zosterops palpebrosus.
Only species for which sequence data are available are included (tree from ref. 31). The range of Z. palpebrosus (light red) overlaps with members of the clade containing the lower 5 species, for example, with Z. japonicus (which is migratory) (light green) in eastern China and Z. atricapilla (red) and Z. montanus (blue) in Indonesia, where Z. palpebrosus is altitudinally segregated from them. Within the Z. palpebrosus clade, all species are allopatric replacements, except for the two species on the Mascarene Islands (bracketed). The timeline is from ref. 31.
Extended Data Figure 3 Plot of lineage versus time and morphological disparity generated in a simple model of ecological controls32.
In this model, new niches appear uniformly through time, and new species arise to fill them, with the criterion that new species are always derived from the ecologically most similar form5,32. For this simulation, the position of a new niche was drawn from a bivariate normal (x, y) with a correlation of 0.5, with 380 niches appearing sequentially and uniformly spaced in time. The result is a linear accumulation of species through time (that is, a downturn on the log scale), and most of the morphological variation accumulating early in the radiation (in the plot, disparity for one variable is shown).
Extended Data Figure 4 Disparity plots for morphology and habitat, with the null Brownian-motion model added.
The large shaded area represents the 95% confidence limit from 100 simulations on 100 trees drawn from the posterior distribution of the Bayesian analysis. The shaded areas around the data plots gives the 95% confidence limits based on phylogenetic uncertainty (based on the same 100 trees as above).
Extended Data Figure 5 Slowdowns in morphological evolution across the tree of the east Himalayan oscines.
a, Maximum likelihood breakpoints (the point in time at which one rate becomes favoured over the other) and changes in rate for two-rate models of morphological evolution. Significance values (*P < 0.01, **P < 0.001) refer to likelihood ratio tests comparing the one- and two-rate Brownian motion models (PC2, P = 0.16). 95% support limits (parentheses) were derived from likelihood profiles averaged across 100 trees sampled from the posterior distribution of Bayesian trees. b, The likelihood profile for evolution of the first shape index (PC1). The likelihood for each (x, y) combination was obtained as the average across 100 trees, then log-transformed for the figure. Numbers are the difference in log-likelihood from the maximum (×100). Only values less than 2 units are shown. The profiles are indicated by symbols (squares for the breakpoint, and circles for the rate difference). c, Relative weights of Ornstein–Uhlenbeck (OU) and Brownian-motion models of morphological evolution at different timelines, based on phylogenetically corrected principal components (revellePCs) (see text). d, Correlations of PC scores with the original (log-transformed) variables.
Extended Data Figure 6 Plot of lineage diversity (on a linear scale) versus time for a phylogeny connecting all species present at 500 m and at 3,000 m.
Eighty-two species are estimated to straddle each of these elevational bands.
Extended Data Figure 7 Morphology at specified elevations.
Grey lines are the convex hull for all species in the study area (points as in Fig. 2). Black lines are the convex hulls for all species whose elevational ranges include the specified band. Blue lines are the convex hulls for all the species censused on 5-hectare grids at those elevations (see the source data, in order of elevation, B2, A3, B1 and G1), and green lines are the convex hulls for all common (>5 pairs per hectare) species on those grids. Number of species is the number of all bird species in that elevational belt, plus (in parentheses) the number of songbirds censused on the grid.
Extended Data Figure 8 Climate data (from http://worldclim.org33).
The top panel shows precipitation mapped on to a topographical map of the study area, showing the locations of the 18 grids. The bottom panels show three predicted climate variables (minimum and maximum temperatures, and precipitation) for the 18 grids. Lines are least-squares regression slopes.
Extended Data Figure 9 Mid-elevation peak plots.
The left panel shows area in 500-m bands between 200 m and 3,700 m in the study area (computed using http://worldclim.org altitude data). The right panel shows the number of oscines in the censused 5-hectare grids and number of oscines in those grids discounted by possible sink species (rare species at the edge of their range).
Supplementary information
Supplementary Information
This file contains Supplementary Table 1. (PDF 152 kb)
Rights and permissions
About this article
Cite this article
Price, T., Hooper, D., Buchanan, C. et al. Niche filling slows the diversification of Himalayan songbirds. Nature 509, 222–225 (2014). https://doi.org/10.1038/nature13272
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13272
This article is cited by
-
The formation of the Indo-Pacific montane avifauna
Nature Communications (2023)
-
Niche expansion and adaptive divergence in the global radiation of crows and ravens
Nature Communications (2022)
-
First steps to success: identification of divergence among the northern and the southern lineages of African Pygmy Kingfisher (Ispidina picta) (Coraciiformes: Alcedinidae)
Journal of Ornithology (2022)
-
Phylogeography and population genetic structure of red muntjacs: evidence of enigmatic Himalayan red muntjac from India
BMC Ecology and Evolution (2021)
-
Climate Change Impacts on Himalayan Biodiversity: Evidence-Based Perception and Current Approaches to Evaluate Threats Under Climate Change
Journal of the Indian Institute of Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.