Abstract
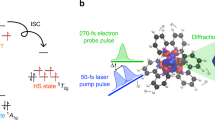
Crucial to many light-driven processes in transition metal complexes is the absorption and dissipation of energy by 3d electrons1,2,3,4. But a detailed understanding of such non-equilibrium excited-state dynamics and their interplay with structural changes is challenging: a multitude of excited states and possible transitions result in phenomena too complex to unravel when faced with the indirect sensitivity of optical spectroscopy to spin dynamics5 and the flux limitations of ultrafast X-ray sources6,7. Such a situation exists for archetypal polypyridyl iron complexes, such as [Fe(2,2′-bipyridine)3]2+, where the excited-state charge and spin dynamics involved in the transition from a low- to a high-spin state (spin crossover) have long been a source of interest and controversy6,7,8,9,10,11,12,13,14,15. Here we demonstrate that femtosecond resolution X-ray fluorescence spectroscopy, with its sensitivity to spin state, can elucidate the spin crossover dynamics of [Fe(2,2′-bipyridine)3]2+ on photoinduced metal-to-ligand charge transfer excitation. We are able to track the charge and spin dynamics, and establish the critical role of intermediate spin states in the crossover mechanism. We anticipate that these capabilities will make our method a valuable tool for mapping in unprecedented detail the fundamental electronic excited-state dynamics that underpin many useful light-triggered molecular phenomena involving 3d transition metal complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gust, D., Moore, T. A. & Moore, A. L. Mimicking photosynthetic solar energy transduction. Acc. Chem. Res. 34, 40–48 (2001)
Sato, O., Iyoda, T., Fujishima, A. & Hashimoto, K. Photoinduced magnetization of a cobalt-iron cyanide. Science 272, 704–705 (1996)
Ferrere, S. & Gregg, B. A. Photosensitization of TiO2 by Fe-II(2,2′-bipyridine-4,4′-dicarboxylic acid)2(CN)2: band selective electron injection from ultra-short-lived excited states. J. Am. Chem. Soc. 120, 843–844 (1998)
Heyduk, A. F. & Nocera, D. G. Hydrogen produced from hydrohalic acid solutions by a two-electron mixed-valence photocatalyst. Science 293, 1639–1641 (2001)
Goldbeck, R. A., Kim-Shapiro, D. B. & Kliger, D. S. Fast natural and magnetic circular dichroism spectroscopy. Annu. Rev. Phys. Chem. 48, 453–479 (1997)
Bressler, C. et al. Femtosecond XANES study of the light-induced spin crossover dynamics in an iron(II) complex. Science 323, 489–492 (2009)
Huse, N. et al. Femtosecond soft X-ray spectroscopy of solvated transition-metal complexes: deciphering the interplay of electronic and structural dynamics. J. Phys. Chem. Lett. 2, 880–884 (2011)
Gutlich, P. & Goodwin, H. A. in Spin Crossover in Transition Metal Compounds I Vol. 233, Topics in Current Chemistry (eds Gutlich, P. & Goodwin, H. A. ) 1–47 (Springer, 2004)
Creutz, C., Chou, M., Netzel, T. L., Okumura, M. & Sutin, N. Lifetimes, spectra, and quenching of the excited-states of polypyridine complexes of iron(II), ruthenium(II), and osmium(II). J. Am. Chem. Soc. 102, 1309–1319 (1980)
Hauser, A. Intersystem crossing in the Fe(PTZ)6 (BF4)2 spin crossover system (PTZ = 1-propyltetrazole). J. Chem. Phys. 94, 2741–2748 (1991)
McCusker, J. K. et al. Subpicosecond 1MLCT-5T2 intersystem crossing of low-spin polypyridyl ferrous complexes. J. Am. Chem. Soc. 115, 298–307 (1993)
Monat, J. E. & McCusker, J. K. Femtosecond excited-state dynamics of an iron(II) polypyridyl solar cell sensitizer model. J. Am. Chem. Soc. 122, 4092–4097 (2000)
Gawelda, W. et al. Ultrafast nonadiabatic dynamics of [Fe(II)(bpy)3]2+ in solution. J. Am. Chem. Soc. 129, 8199–8206 (2007)
Consani, C. et al. Vibrational coherences and relaxation in the high-spin state of aqueous [Fe-II(bpy)3]2+. Angew. Chem. Int. Edn 48, 7184–7187 (2009)
Lemke, H. T. et al. Femtosecond X-ray absorption spectroscopy at a hard X-ray free electron laser: application to spin crossover dynamics. J. Phys. Chem. A 117, 735–740 (2013)
Emma, P. et al. First lasing and operation of an angstrom-wavelength free-electron laser. Nature Photon. 4, 641–647 (2010)
Harmand, M. et al. Achieving few-femtosecond time-sorting at hard X-ray free-electron lasers. Nature Photon. 7, 215–218 (2013)
Haldrup, K. et al. Guest-host interactions investigated by time-resolved X-ray spectroscopies and scattering at MHz rates: solvation dynamics and photoinduced spin transition in aqueous [Fe(bipy)3]2+. J. Phys. Chem. A 116, 9878–9887 (2012)
Vankó, G. et al. Probing the 3d spin momentum with X-ray emission spectroscopy: the case of molecular-spin transitions. J. Phys. Chem. B 110, 11647–11653 (2006)
Krause, M. O. & Oliver, J. H. Natural widths of atomic K and L levels, K-alpha X-ray lines and several KLL Auger lines. J. Phys. Chem. Ref. Data 8, 329–338 (1979)
de Graaf, C. & Sousa, C. Study of the light-induced spin crossover process of the [Fe(II)(bpy)3]2+ complex. Chemistry 16, 4550–4556 (2010)
de Graaf, C. & Sousa, C. On the role of the metal-to-ligand charge transfer states in the light-induced spin crossover in Fe-II(bpy)3 . Int. J. Quantum Chem. 111, 3385–3393 (2011)
Glatzel, P. & Bergmann, U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes — electronic and structural information. Coord. Chem. Rev. 249, 65–95 (2005)
de Groot, F. High resolution X-ray emission and X-ray absorption spectroscopy. Chem. Rev. 101, 1779–1808 (2001)
Lee, N., Petrenko, T., Bergmann, U., Neese, F. & DeBeer, S. Probing valence orbital composition with iron K β X-ray emission spectroscopy. J. Am. Chem. Soc. 132, 9715–9727 (2010)
Cannizzo, A. et al. Light-induced spin crossover in Fe(II)-based complexes: the full photocycle unraveled by ultrafast optical and X-ray spectroscopies. Coord. Chem. Rev. 254, 2677–2686 (2010)
Sousa, C. et al. Ultrafast deactivation mechanism of the excited singlet in the light-induced spin crossover of [Fe(2,2′-bipyridine)3]2+. Chemistry 19, 17541–17551 (2013)
Alvarez, S. Relationships between temperature, magnetic moment, and continuous symmetry measures in spin crossover complexes. J. Am. Chem. Soc. 125, 6795–6802 (2003)
Khalil, M. et al. Picosecond X-ray absorption spectroscopy of a photoinduced iron(II) spin crossover reaction in solution. J. Phys. Chem. A 110, 38–44 (2006)
Nozawa, S. et al. Direct probing of spin state dynamics coupled with electronic and structural modifications by picosecond time-resolved XAFS. J. Am. Chem. Soc. 132, 61–63 (2010)
Gawelda, W. Time-Resolved X-Ray Absorption Spectroscopy of Transition Metal Complexes. Ph.D. thesis, École Polytechnique Fédérale de Lausanne. (2006)
Feng, Y. P. et al. A single-shot intensity-position monitor for hard X-ray FEL sources. Proc. SPIE 8140, 81400Q (2011)
Alonso-Mori, R. et al. A multi-crystal wavelength dispersive X-ray spectrometer. Rev. Sci. Instrum. 83, 9 (2012)
Koerner, L. J., Philipp, H. T., Hromalik, M. S., Tate, M. W. & Gruner, S. M. X-ray tests of a pixel array detector for coherent X-ray imaging at the Linac Coherent Light Source. J. Instrum. 4, P03001 (2009)
Bionta, M. R. et al. Spectral encoding of X-ray/optical relative delay. Opt. Express 19, 21855–21865 (2011)
Vankó, G. et al. Picosecond time-resolved X-ray emission spectroscopy: ultrafast spin-state determination in an iron complex. Angew. Chem. Int. Edn 49, 5910–5912 (2010)
Vankó, G. et al. Spin-state studies with XES and RIXS: From static to ultrafast. J. Elec. Spec. Relat. Phenom. 188, 166–171 (2013)
de Groot, F. M. F. & Kotani, A. Core Level Spectroscopy of Solids (CRC Press, Boca Raton, 2008)
Stepanow, S. et al. Mixed-valence behavior and strong correlation effects of metal phthalocyanines adsorbed on metals. Phys. Rev. B 83, 220401 (2011)
Kutner, M. H., Nachtsheim, C. J. & Neter, J. Applied Linear Regression Models (McGraw-Hill/Irwin, 2004)
Acknowledgements
We thank P. Frank, B. Lin and S. DeBeer for discussion, S. DeBeer for some model iron complex X-ray fluorescence spectra, and D. Stanbury for providing some iron complexes. Experiments were carried out at LCLS and SSRL, which are National User Facilities operated for DOE and OBES respectively by Stanford University. W.Z., R.W.H., H.W.L., D.A.M., Z.S. and K.J.G. acknowledge support from the AMOS programme within the Chemical Sciences, Geosciences and Biosciences Division of the Office of Basic Energy Sciences, Office of Science, US Department of Energy. E.I.S. acknowledges support from the NSF (CHE-0948211). R.G.H. acknowledges a Gerhard Casper Stanford Graduate Fellowship and the Achievements Rewards for College Scientists (ARCS) Foundation. T.K. acknowledges the German Research Foundation (DFG), grant KR3611/2-1. K.S.K., M.M.N. and T.B.v.D. acknowledge support from the Danish National Research Foundation and from DANSCATT. K.K. thanks the Volkswagen Foundation for support under the Peter Paul Ewald fellowship program (I/85832). G.V. acknowledges support from the European Research Council (ERC-StG-259709) and the Lendület Programme of the Hungarian Academy of Sciences. C.B., W.G. and A.G. thank the DFG (SFB925), as well as the European XFEL, for financial support.
Author information
Authors and Affiliations
Contributions
W.Z., R.A.-M., U.B., R.W.H., D.A.M., T.-C.W. and K.J.G. designed the experiment. W.Z., R.A.-M., U.B., M.C., R.W.H., K.S.K., K.K., H.T.L., H.W.L., C.P., J.S.R., Z.S., D.S., T.B.v.D., T.-C.W., D.Z. and K.J.G. did the experiment. W.Z., T.K., K.S.K., T.B.v.D., G.V. and T.-C.W. analysed the data. W.Z., R.A.-M., U.B., C.B., W.G., A.G., R.G.H., R.W.H., T.K., K.S.K., K.K., D.A.M., M.M.N., E.I.S., D.S. and K.J.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
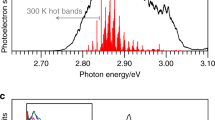
Extended Data Figure 1 Experimental and calculated Kβ fluorescence spectra for triplet spin states.
a, The calculated Kβ fluorescence spectra of iron complexes: triplet Fe(ii) in square planar crystal field (red) (calculation parameters based on Fe(ii)phthalocyanine), and triplet excited state in an octahedral crystal field (blue) (calculation parameters based on [Fe(2,2′-bipyridine)3]2+). b, The experimental Kβ fluorescence difference spectrum (red) obtained by subtracting the singlet [Fe(2,2′-bipyridine)3]2+ spectrum from the triplet Fe(ii)phthalocyanine spectrum, and the calculated Kβ fluorescence difference spectrum (blue) generated by subtracting the spectrum of the singlet state in an octahedral crystal field from the triplet state in a square planar crystal field.
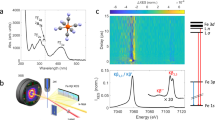
Extended Data Figure 2 Time-dependent Kβ fluorescence spectra and fit using the sequential kinetic model with a triplet transient.
a, Experimental transient fluorescent amplitude difference spectra plotted with arbitrary units, and b, fit using the sequential kinetic model with a triplet transient. c, Residuals for the best fit, with the colour-scale maximum and minimum set to one-fifth of the value used in a and b. d, The excited state populations extracted from the best fit.
Extended Data Figure 3 Time-dependent Kβ fluorescence spectra and fit using the direct kinetic model without a triplet transient.
a, Experimental transient fluorescent amplitude difference spectra plotted with arbitrary units, and b, fit using the direct kinetic model without a triplet transient. c, Residuals for the best fit with the colour scale maximum and minimum set to one-fifth of the value used in a and b. d, The excited state populations extracted from the best fit.
Extended Data Figure 4 The 50 fs time delay normalized Kβ fluorescent amplitude difference spectrum (ΔI) and kinetic model fit plotted as a function of X-ray emission energy.
The measured data (black circles and line), along with the best global fit from the sequential kinetic model with a transient triplet state (red line).
Extended Data Figure 5 Absorption spectrum and pump power dependence measurements.
a, The ultraviolet–visible absorption spectrum of [Fe(2,2′-bipyridine)3]2+ in water. b, Power (fluence) dependence of the change in probe transmission measured at 520 nm, following excitation of an aqueous solution of [Fe(2,2′-bipyridine)3]Cl2 with a 520 nm pump pulse. The figure shows the change in transmission (ΔT) measured at a 10 ps time delay, a time long compared to the spin crossover and vibrational cooling timescales, but short compared to the lifetime of the high-spin excited state.
Source data
Rights and permissions
About this article
Cite this article
Zhang, W., Alonso-Mori, R., Bergmann, U. et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 509, 345–348 (2014). https://doi.org/10.1038/nature13252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13252
This article is cited by
-
Ferricyanide photo-aquation pathway revealed by combined femtosecond Kβ main line and valence-to-core x-ray emission spectroscopy
Nature Communications (2023)
-
Spin–vibronic coherence drives singlet–triplet conversion
Nature (2023)
-
Branching mechanism of photoswitching in an Fe(II) polypyridyl complex explained by full singlet-triplet-quintet dynamics
Communications Chemistry (2023)
-
Progress and prospects in nonlinear extreme-ultraviolet and X-ray optics and spectroscopy
Nature Reviews Physics (2023)
-
Photoredox-active Cr(0) luminophores featuring photophysical properties competitive with Ru(II) and Os(II) complexes
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.