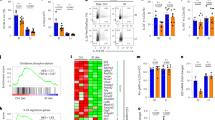
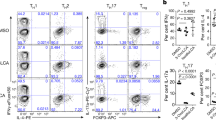
Abstract
The impact of nutritional status during fetal life on the overall health of adults has been recognized1; however, dietary effects on the developing immune system are largely unknown. Development of secondary lymphoid organs occurs during embryogenesis and is considered to be developmentally programmed2,3. Secondary lymphoid organ formation depends on a subset of type 3 innate lymphoid cells (ILC3) named lymphoid tissue inducer (LTi) cells2,3,4,5. Here we show that mouse fetal ILC3s are controlled by cell-autonomous retinoic acid (RA) signalling in utero, which pre-sets the immune fitness in adulthood. We found that embryonic lymphoid organs contain ILC progenitors that differentiate locally into mature LTi cells. Local LTi cell differentiation was controlled by maternal retinoid intake and fetal RA signalling acting in a haematopoietic cell-autonomous manner. RA controlled LTi cell maturation upstream of the transcription factor RORγt. Accordingly, enforced expression of Rorgt restored maturation of LTi cells with impaired RA signalling, whereas RA receptors directly regulated the Rorgt locus. Finally, we established that maternal levels of dietary retinoids control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring. Our results reveal a molecular link between maternal nutrients and the formation of immune structures required for resistance to infection in the offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gluckman, P. D. & Hanson, M. A. Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736 (2004)
van de Pavert, S. A. & Mebius, R. E. New insights into the development of lymphoid tissues. Nature Rev. Immunol. 10, 664–674 (2010)
Randall, T. D., Carragher, D. M. & Rangel-Moreno, J. Development of secondary lymphoid organs. Annu. Rev. Immunol. 26, 627–650 (2008)
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3- LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997)
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nature Immunol. 5, 64–73 (2004)
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s Patch organogenesis. Nature 446, 547–551 (2007)
Patel, A. et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal. 5, ra55 (2012)
Cupedo, T. et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J. Immunol. 173, 2968–2975 (2004)
Cherrier, M., Sawa, S. & Eberl, G. Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J. Exp. Med. 209, 729–740 (2012)
van de Pavert, S. A. et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nature Immunol. 10, 1193–1199 (2009)
Niederreither, K. & Dolle, P. Retinoic acid in development: towards an integrated view. Nature Rev. Genet. 9, 541–553 (2008)
Iwata, M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 21, 8–13 (2009)
Hall, J. A. et al. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor alpha. Immunity 34, 435–447 (2011)
Mora, J. R. & von Andrian, U. H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin. Immunol. 21, 28–35 (2009)
Mucida, D. et al. Retinoic acid can directly promote TGF-β-mediated Foxp3+Treg cell conversion of naive T cells. Immunity 30, 471–472; Reply. 472–473 (2009)
Hall, J. A., Grainger, J. R., Spencer, S. P. & Belkaid, Y. The role of retinoic acid in tolerance and immunity. Immunity 35, 13–22 (2011)
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003)
Rosselot, C. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010)
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000)
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999)
Aliahmad, P., de la Torre, B. & Kaye, J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nature Immunol. 11, 945–952 (2010)
Possot, C. et al. Notch signaling is necessary for adult, but not fetal, development of RORγt+ innate lymphoid cells. Nature Immunol. 12, 949–958 (2011)
Tachibana, M. et al. Runx1/Cbfβ2 complexes are required for lymphoid tissue inducer cell differentiation at two developmental stages. J. Immunol. 186, 1450–1457 (2011)
Meier, D. et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654 (2007)
Gredmark-Russ, S., Cheung, E. J., Isaacson, M. K., Ploegh, H. L. & Grotenbreg, G. M. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J. Virol. 82, 12205–12212 (2008)
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011)
Lee, J. S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nature Immunol. 13, 144–151 (2011)
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2011)
Karrer, U. et al. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185, 2157–2170 (1997)
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014)
Rawlins, E. L., Clark, C. P., Xue, Y. & Hogan, B. L. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136, 3741–3745 (2009)
Eberl, G. & Littman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004)
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001)
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007)
de Bruijn, M. F. et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16, 673–683 (2002)
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994)
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992)
Niederreither, K. et al. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019–1031 (2001)
Veiga-Fernandes, H., Foster, K., Patel, A., Coles, M. & Kioussis, D. Visualisation of lymphoid organ development. Methods Mol. Biol. 616, 161–179 (2010)
Huh, J. R. et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 (2011)
Sunil-Chandra, N. P., Efstathiou, S., Arno, J. & Nash, A. A. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73, 2347–2356 (1992)
Weck, K. E., Barkon, M. L., Yoo, L. I., Speck, S. H. & Virgin, H. I. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70, 6775–6780 (1996)
Acknowledgements
We thank the imaging, animal and flow cytometry facilities at IMM and UPC for technical assistance; C. Mendelsohn for providing ROSA26-RARα403 mice; N. Schmolka, J. G. van Rietschoten, R. E. van Kesteren, T. H. B. Geijtenbeek, S. Gringhuis, E. Keuning, J. Peterson-Maduro, M. G. Roukens, D. D’Astolfo, M. Vermunt, A. Rijerkerk, J. Koning, J. van der Meulen and B. Oliver for technical help; and G. Vilhais-Neto, M. C. Coles and G. Eberl for discussion. M.F., L.M.-S. and R.G.D. were supported by FCT, Portugal; H.V.-F. by EMBO (1648) and ERC (207057); D.R.L. by NIH (RO1AI080885) and HHMI; M.R.M. by Dutch MS research foundation (MS 12-797); S.A.vd.P. by NGI Breakthrough Horizon (40-41009-98-9077); and R.E.M. by a VICI (918.56.612) and ALW-TOP grant (09.048).
Author information
Authors and Affiliations
Contributions
M.F. wrote the manuscript, designed, performed and analysed the experiments in Figs 1a, b, d, e, g–i, 2a–h, 3a, b, d–i and 4a, b, d–j and Extended Data Figs 1a, d–f, 2a–d, 3a–h, 4a–d, 5a–c, 6, 7a–f, 8a–d, 9a, b and 10a–c. S.A.vd.P. wrote the manuscript, designed, performed and analysed the experiments in Figs 1c, f, 3c, g–i and 4c and Extended Data Figs 1b, c, e, 5b, c and 6. R.G.D., H.R., R.M., L.M.-S., F.F.A., S.I., I.B., G.G., C.L.-A., T.K., D.S., T.O’T., M.R.M., Y.H. and S.S-M. contributed to several experiments. C.G.-S. and J.P.S. provided murid herpesvirus-4. D.R.L. and F.R.S provided Rorgt−/− embryos. E.H. and E.D. provided Ly-6A (Sca1)-GFP mice. R.E.M. and H.V.-F. supervised the work, planned the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Fetal ILCs.
a, ILC subsets in fetal gut and lymph nodes (LNs). b, E15.5 intestines and lymph node cells were purified from Id2GFP and wild-type mice. Id2GFP and RORγt expression are shown in ILC4neg (CD3−IL-7Rα+α4β7+ID2+c-Kit+CD11c−CD4−) and LTi4 (CD3−IL-7Rα+α4β7+ID2+c-Kit+CD11c−RORγt+CD4+) cells. c, E13.5 and E14.5 Ly6A-GFP anlagen lymph nodes were stained with GFP, IL-7Rα and Ki67 antibodies and analysed by confocal microscopy. d, E14.5 Rorgt−/− mesenteric lymph nodes were stained with podoplanin, IL-7Rα and CD45 antibodies and analysed by confocal microscopy. e, Percentage of E16.5 ILC4neg and LTi4 cells gated in CD45+CD3−CD11c− determined by flow cytometry in Rorgt+/+ and Rorgt−/− intestines. Data are representative of three independent experiments. f, Left: pregnant mice received RAR antagonist BMS493 or the vehicle DMSO from E10.5 until E13.5. The ratio LTi4/ILC4neg cells in the fetal liver was determined at E13.5; n = 8. Right: pregnant mice received BMS493 or the vehicle DMSO. Frequency of colonizing haematopoietic cells was determined in E17.5 intestines by flow cytometry; n = 4. g, Pregnant hCD2-GFP mice were administered BMS493 or DMSO from E10.5 until E13.5. Embryos were analysed at E17.5; n = 13. Arrowheads show anlagen lymph nodes. Scale bars: 50 μm (c, d); 500 μm (g). Error bars show s.e. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 2 Analysis of mouse haematopoietic-cell-specific Cre lines.
a, Vav-iCre and hCD2-Cre mice were crossed with ROSA26-Tomato-mGFP mice. Rorgt-Cre and Id2-CreERT2 mice were crossed with ROSA26-eYFP mice. E15.5 intestines were analysed by flow cytometry. Left: results show the percentage of mGFP or eYFP positive cells in gut cell suspensions. Right: percentage of mGFP or eYFP positive cells in non-haematopoietic (CD45−), haematopoietic (CD45+), LTi and LTin cells. Results are representative of three independent experiments. b, Percentage of enteric E15.5 ILC4neg and LTi4 cells determined by flow cytometry in wild-type, Rorgt-Cre RarHet and Rorgt-Cre RarHom littermates. c, Frequencies of enteric ILC4neg and LTi4 cells in wild-type, Rorgt-Cre RarHet and Rorgt-Cre RarHom littermates. WT, n = 5; Rorgt-Cre RarHet, n = 5; Rorgt-Cre RarHom, n = 4. d, Peyer’s patch area at 6–7 weeks of age. WT, n = 3; Rorgt-Cre RarHet, n = 4. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 3 Analysis of Vav-iCre/ROSA26-RARα403 mice.
a, E15.5 intestines from wild-type, Vav-iCre RarHet and RarHom mice were analysed by flow cytometry. Representative analysis of six independent experiments is shown. b, E15.5 regions of cervical, brachial and inguinal lymph node from wild-type, Vav-iCre RarHet and RarHom mice were analysed by flow cytometry. Representative analysis of two independent experiments. c, LTin cell percentage and LTi4/ILC4neg cell ratios are shown in E15.5 lymph nodes; n = 4. d, E15.5 fetal livers from wild-type and Vav-iCre RarHet mice were analysed by flow cytometry. Results show number of CD45+Lin− (n = 4) and CD45−Lin−IL-7Rα+α4β7+ progenitors (n = 3). e, Percentage of CD45+CD3−CD11c−IL-7Rα+RORγt+CD4− (RORγt+CD4−) and CD45+CD3−CD11c−IL-7Rα+RORγt+CD4+ (RORγt+CD4+) cells determined by flow cytometry in RARHet and wild-type littermate controls in E15.5 guts and lymph nodes. f, Frequencies of RORγt+CD4− and RORγt+CD4+ cells in mice described in e; WT, n = 9; RarHet, n = 3. g, ILC4neg cells were purified from E15.5 wild-type intestines by flow cytometry and cultured for 6 days. LTi4 cells raised in vitro were purified by flow cytometry and quantitative RT–PCR analysis performed. Results show log2 fold increase in comparison to their cultured ILC4neg cell counterparts. Results were normalized to Hprt1 and Gapdh. h, Left: E15.5 embryos were whole-mount stained for CD4 (red) and imaged by confocal microscopy. Cervical (Cer) lymph nodes are shown. Right: cervical lymph node dimensions are shown. WT, n = 5; RarHet, n = 7; RarHom, n = 6. Scale bar: 50 μm. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant. ND, not detected.
Extended Data Figure 4 Gene expression patterns in ILC4neg and LTi4 cells.
a, E15.5 intestines from RarHom and wild-type littermate controls were brought to suspension and analysed by flow cytometry. Upper panel: RORγt expression. Lower panel: mean fluorescence intensity of RORγt expression in ILC4neg cells; n = 3. b, ILC4neg cells were purified from E15.5 RarHet and wild-type littermate control intestines and lymph nodes. Quantitative RT–PCR analysis was performed. Results show log2 fold increase to wild type. Results were normalized to Hprt1 and Gapdh. Results from three independent measurements are shown. c, d, LTi4 cells were purified from E15.5 RarHet (c), RarHom (d) and wild-type littermate control intestines and lymph nodes. Quantitative RT–PCR analysis was performed. Results show log2 fold increase to wild type. Results were normalized to Hprt1 and Gapdh. Data from three independent measurements are shown. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. ND, not detected.
Extended Data Figure 5 Treatment of ILC4neg and LTi4 cells with digoxin.
a, Wild-type ILC4neg cells were FACS purified, starved overnight and stimulated with DMSO, BMS493 (100 nM), RA (100 nM) and RA plus BMS493 (100 nM each) for 16 h. Results show quantitative RT–PCR analysis normalized to Gapdh; n = 3. b, E13.5 lymph node cell suspensions were cultured with vehicle (ethanol), digoxin, digoxin + RA and RA alone for 24 h. Alive/dead cell ratios were determined by flow cytometry and DAPI staining; n = 4. c, ILC4neg cells were isolated from wild-type E15.5 embryos starved overnight and stimulated for 6 h in the presence of RA (100 nM) or DIG (10 µM) + RA (100 nM). Results show quantitative RT–PCR analysis of Rorgt and RORγt downstream targets normalized to Gapdh. Representative of three independent experiments. Error bars show s.e. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 6 Analysis of SLOs from adult mice with variable RA signalling levels.
a, Axillary (Axi), brachial (Bra) and inguinal (Ing) lymph nodes from adult RarHet and wild-type littermate controls were analysed. Results show lymph node cell numbers; n = 6. b, Results show Peyer’s patch number per intestine from adult RarHet and wild-type littermate controls; n = 6. c, Six-week-old wild-type females received VAC, VAH or VAD (n = 3) diet for 7 weeks. Axillary (Axi), brachial (Bra), inguinal (Ing), intrathoracic (IntraT) lymph nodes and Peyer’s patches (PP) dimensions were analysed; n = 3. d, Percentage of CD45+CD19+ B cells; CD4+ and CD8+ T cells in inguinal lymph nodes; n = 3. e, f, Two-week-old CD45.2 RarHet and wild-type littermate controls were lethally irradiated and transplanted with wild-type CD45.1 bone marrow cells. Chimaeric mice were analysed 8 weeks after reconstitution. e, Results show Peyer’s patch dimensions and follicle number/Peyer’s patch; n = 6. f, Results show number of cells in axillary (Axi), brachial (Bra) inguinal (Ing) and intrathoracic (IntraT) lymph nodes; n = 6. Scale bar: 1 mm. Error bars show s.e. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 7 Analysis of WT→WT and WT→RarHet bone marrow chimaeras.
Two-week-old CD45.2 RarHet and wild-type littermate controls were lethally irradiated and transplanted with wild-type CD45.1 bone marrow cells. Chimaeric mice were analysed 8 weeks after reconstitution. a, Reconstitution of donor CD45.1 cells in WT→WT and WT→RarHet chimaeras in the spleen (n = 4). b, Reconstitution of donor CD45.1 CD4 and CD8 T cells in WT→WT and WT→RarHet chimaeras in the spleen. WT→WT, n = 4; WT→RarHet, n = 11. c, Reconstitution of donor CD45.1 CD11c+MHCII+ and CD11b+Gr1+ myeloid cells in WT→WT and WT→RarHet chimaeras in the spleen; n = 3. d, Reconstitution of donor CD45.1 CD11c+MHCII+ and CD11b+Gr1+ cells in WT→WT and WT→RarHet chimaeras in intrathoracic lymph nodes; n = 3. e, Dendritic cells (DCs) were purified from WT→WT and WT→RarHet chimaeras. Dendritic cells were loaded with OVA peptide (10−5 μM) and co-cultured for 3 days with CFSE-labelled monoclonal OT1 CD8 T cells. OT1 CD8 T-cell proliferation was analysed by CFSE dilution. Proliferation index is shown. WT, n = 4; RarHet, n = 6. f, Dendritic cells were purified from WT→WT and WT→RarHet chimaeras. Dendritic cells were loaded with OVA peptide (10−5 μM) and co-cultured for 3 days with OT1 CD8 T cells. Percentage of IFN-γ-producing OT1 CD8 T cells is shown; n = 4. Error bars show s.e. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 8 Infection of WT→RarHet or WT→WT chimaeras with murid herpesvirus-4.
a, Intrathoracic lymph node cellularity in WT→WT and WT→RarHet chimaeras at different days post-infection (d.p.i.). WT, n = 5; RarHet, n = 3. b, Percentage of CD8+ORF61+ (left) and ORF75c+ (right) T cells in intrathoracic lymph nodes at different days post-infection (d.p.i.). WT, n = 5; RarHet, n = 3. c, Percentage of donor CD45.1 CD8+ORF61+ (left) and CD8+ORF75c+ (right) T cells in WT→WT and WT→RarHet chimaeras after infection with murid herpesvirus-4. WT, n = 6; RarHet, n = 12. Error bars show s.e. Two-tailed t-test P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
Extended Data Figure 9 Impact of maternal retinoids in ILC3s.
Maternal dietary intake of vitamin A is catabolized into bioactive retinoic acid (RA). RA signals control type 3 innate lymphoid cells (ILC3) in the embryo. Fetal ILC3s include ILC4neg and LTi4 cells. LTi4 cells are Id2+RORγt+, whereas enteric ILC4neg cells contain a minor subset of Id2+RORγt− cells (pre-ILCs). RA signalling operates in a cell-autonomous fashion, via direct regulation of Rorgt, programming innate pro-inflammatory cytokines and chemokines and differentiation of LTi4 cells.
Rights and permissions
About this article
Cite this article
van de Pavert, S., Ferreira, M., Domingues, R. et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508, 123–127 (2014). https://doi.org/10.1038/nature13158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13158
This article is cited by
-
Disease pathogenesis and barrier functions regulated by group 3 innate lymphoid cells
Seminars in Immunopathology (2024)
-
Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease
Nature Reviews Immunology (2023)
-
Lymphoid tissue inducer cells in cancer: a potential therapeutic target
Molecular and Cellular Biochemistry (2023)
-
Composition and dynamics of intestinal fungi during the postnatal 2 months of very low birth weight infants
European Journal of Pediatrics (2023)
-
Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta
BMC Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.