Abstract
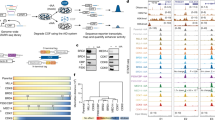
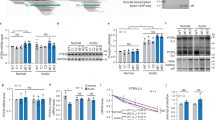
Rev-Erb-α and Rev-Erb-β are nuclear receptors that regulate the expression of genes involved in the control of circadian rhythm1,2, metabolism3,4 and inflammatory responses5. Rev-Erbs function as transcriptional repressors by recruiting nuclear receptor co-repressor (NCoR)–HDAC3 complexes to Rev-Erb response elements in enhancers and promoters of target genes6,7,8, but the molecular basis for cell-specific programs of repression is not known. Here we present evidence that in mouse macrophages Rev-Erbs regulate target gene expression by inhibiting the functions of distal enhancers that are selected by macrophage-lineage-determining factors, thereby establishing a macrophage-specific program of repression. Remarkably, the repressive functions of Rev-Erbs are associated with their ability to inhibit the transcription of enhancer-derived RNAs (eRNAs). Furthermore, targeted degradation of eRNAs at two enhancers subject to negative regulation by Rev-Erbs resulted in reduced expression of nearby messenger RNAs, suggesting a direct role of these eRNAs in enhancer function. By precisely defining eRNA start sites using a modified form of global run-on sequencing that quantifies nascent 5′ ends, we show that transfer of full enhancer activity to a target promoter requires both the sequences mediating transcription-factor binding and the specific sequences encoding the eRNA transcript. These studies provide evidence for a direct role of eRNAs in contributing to enhancer functions and suggest that Rev-Erbs act to suppress gene expression at a distance by repressing eRNA transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002)
Liu, A. C. et al. Redundant function of REV-ERBα and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 4, e1000023 (2008)
Raspé, E. et al. Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J. Lipid Res. 43, 2172–2179 (2002)
Le Martelot, G. et al. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 7, e1000181 (2009)
Gibbs, J. E. et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 582–587 (2012)
Zamir, I. et al. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol. Cell. Biol. 16, 5458–5465 (1996)
Yin, L. & Lazar, M. A. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol. Endocrinol. 19, 1452–1459 (2005)
Feng, D. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011)
Heintzman, N. D. et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459, 108–112 (2009)
Friedman, A. D. Transcriptional control of granulocyte and monocyte development. Oncogene 26, 6816–6828 (2007)
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010)
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008)
Schlaeger, T. M., Mikkola, H. K., Gekas, C., Helgadottir, H. B. & Orkin, S. H. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood 105, 3871–3874 (2005)
Giguere, V. et al. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 8, 538–553 (1994)
Kim, T.-K. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 (2010)
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011)
Hah, N. et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145, 622–634 (2011)
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010)
Guang, S. et al. Small regulatory RNAs inhibit RNA polymerase II during the elongation phase of transcription. Nature 465, 1097–1101 (2010)
Gu, S. G. et al. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nature Genet. 44, 157–164 (2012)
Huang, W. et al. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature 470, 414–418 (2011)
Lai, F. et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494, 497–501 (2013)
Melo, C. A. et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol. Cell 49, 524–535 (2013)
Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010)
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012)
Saldanha, A. J. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Schanke, J. T. Sequence inversion by Flip-PCR. Methods Mol. Biol. 67, 203–208 (1997)
Baker, B. F. et al. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 272, 11994–12000 (1997)
Seth, P. P. et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp. Ser. 52, 553–554 (2008)
Acknowledgements
We thank L. Bautista for assistance with figure preparation. These studies were supported by National Institutes of Health grants CA17390, U19DK62434, DK091183, DK063491, CA014195, DK057978, HL088093, HL105278 and CA52599. M.G.R. and R.M.E. are Investigators of the Howard Hughes Medical Institute. M.T.Y.L. is supported by the University of California, San Diego Medical Scientist Training Program T32 GM007198-37, and Genetics Training Program T32 GM008666, National Institute of General Medical Sciences. M.U.K. was supported by a LeDucq Foundation Fellowship. H.P.L. was supported by the Finnish Cultural Foundation, Instumentarium Foundation, The Paulo Foundation, Paavo Nurmi Foundation, Finnish Foundation for Cardiovascular Research, The Maud Kuistila Memorial Foundation and The Fulbright Center. These studies were also supported by grants from the Leona M. and Harry B. Helmsley Charitable Trust, Samuel Waxman Cancer Research Foundation, the Glenn Foundation for Medical Research, the Ellison Medical Foundation and Ipsen/Biomeasure.
Author information
Authors and Affiliations
Contributions
M.T.Y.L., S.H., C.B., A.W., T.R.G., M.G.R., R.M.E. and C.K.G. conceived the project and planned experiments, which were performed by M.T.Y.L., H.C., H.P.L., D.G., S.H., Y.T.-O., M.U.K., A.S.K., M.K. and C.Y.L., and analysed by M.T.Y.L., H.P.L., D.G., C.B. and C.K.G. The project was supervised by C.K.G., who wrote the manuscript with M.T.Y.L.
Corresponding author
Ethics declarations
Competing interests
A.S.K., A.W. and T.R.G. are employees of Isis Pharmaceuticals.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-16 and additional references. (PDF 12417 kb)
Rights and permissions
About this article
Cite this article
Lam, M., Cho, H., Lesch, H. et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 498, 511–515 (2013). https://doi.org/10.1038/nature12209
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12209
This article is cited by
-
Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets
Cancer and Metastasis Reviews (2023)
-
The enhancer RNA ADCY10P1 is associated with the progression of ovarian cancer
Journal of Ovarian Research (2022)
-
Enhancer RNAs stimulate Pol II pause release by harnessing multivalent interactions to NELF
Nature Communications (2022)
-
Glucocorticoid circadian rhythms in immune function
Seminars in Immunopathology (2022)
-
SREBP2-dependent lipid gene transcription enhances the infection of human dendritic cells by Zika virus
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.