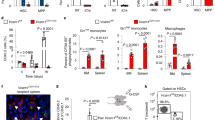
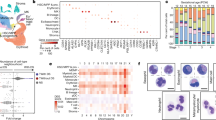
Abstract
How environmental cues regulate adult stem cell and cancer cell activity through surface receptors is poorly understood. Angiopoietin-like proteins (ANGPTLs), a family of seven secreted glycoproteins, are known to support the activity of haematopoietic stem cells (HSCs) in vitro and in vivo1,2,3,4,5,6,7,8,9,10. ANGPTLs also have important roles in lipid metabolism, angiogenesis and inflammation, but were considered ‘orphan ligands’ because no receptors were identified3,11,12. Here we show that the immune-inhibitory receptor human leukocyte immunoglobulin-like receptor B2 (LILRB2) and its mouse orthologue paired immunoglobulin-like receptor (PIRB) are receptors for several ANGPTLs. LILRB2 and PIRB are expressed on human and mouse HSCs, respectively, and the binding of ANGPTLs to these receptors supported ex vivo expansion of HSCs. In mouse transplantation acute myeloid leukaemia models, a deficiency in intracellular signalling of PIRB resulted in increased differentiation of leukaemia cells, revealing that PIRB supports leukaemia development. Our study indicates an unexpected functional significance of classical immune-inhibitory receptors in maintenance of stemness of normal adult stem cells and in support of cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, C. C., Kaba, M., Iizuka, S., Huynh, H. & Lodish, H. F. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 111, 3415–3423 (2008)
Zhang, C. C. et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nature Med. 12, 240–245 (2006)
Zhang, C. C. & Lodish, H. F. Cytokines regulating hematopoietic stem cell function. Curr. Opin. Hematol. 15, 307–311 (2008)
Huynh, H. et al. IGFBP2 secreted by a tumorigenic cell line supports ex vivo expansion of mouse hematopoietic stem cells. Stem Cells 26, 1628–1635 (2008)
Chou, S. & Lodish, H. F. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl Acad. Sci. USA 107, 7799–7804 (2010)
Lin, M. & Zon, L. I. Genetic analyses in zebrafish reveal that angiopoietin-like proteins 1 and 2 are required for HSC development during embryogenesis. Am. Soc. Hematol. 50th Ann. Meeting Abstract 729 186 (2008)
Khoury, M. et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 20, 1371–1381 (2011)
Drake, A. C. et al. Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD–SCID Il2rγ−/−(NSG) mice. PLoS ONE 6, e18382 (2011)
Zheng, J., Huynh, H., Umikawa, M., Silvany, R. & Zhang, C. C. Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood 117, 470–479 (2011)
Zheng, J. et al. Ex vivo expanded hematopoietic stem cells overcome the MHC barrier in allogeneic transplantation. Cell Stem Cell 9, 119–130 (2011)
Hato, T., Tabata, M. & Oike, Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc. Med. 18, 6–14 (2008)
Tabata, M. et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 10, 178–188 (2009)
Barrow, A. D. & Trowsdale, J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol. Rev. 224, 98–123 (2008)
Meyaard, L. LAIR and collagens in immune regulation. Immunol. Lett. 128, 26–28 (2010)
Kitsos, C. M. et al. Calmodulin-dependent protein kinase IV regulates hematopoietic stem cell maintenance. J. Biol. Chem. 280, 33101–33108 (2005)
Takai, T., Nakamura, A. & Endo, S. Role of PIR-B in autoimmune glomerulonephritis. J. Biomed. Biotechnol. 2011, 275302 (2011)
Atwal, J. K. et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322, 967–970 (2008)
Syken, J., Grandpre, T., Kanold, P. O. & Shatz, C. J. PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800 (2006)
Chan, R. J. et al. Shp-2 heterozygous hematopoietic stem cells have deficient repopulating ability due to diminished self-renewal. Exp. Hematol. 34, 1229–1238 (2006)
Si, J. & Collins, S. J. Activated Ca2+/calmodulin-dependent protein kinase IIγ is a critical regulator of myeloid leukemia cell proliferation. Cancer Res. 68, 3733–3742 (2008)
Lukk, M. et al. A global map of human gene expression. Nature Biotechnol. 28, 322–324 (2010)
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442, 818–822 (2006)
Somervaille, T. C. & Cleary, M. L. Identification and characterization of leukemia stem cells in murine MLL–AF9 acute myeloid leukemia. Cancer Cell 10, 257–268 (2006)
Lavau, C., Szilvassy, S. J., Slany, R. & Cleary, M. L. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 16, 4226–4237 (1997)
Ma, G. et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 34, 385–395 (2011)
Mori, Y. et al. Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J. Immunol. 181, 4742–4751 (2008)
Chan, R. J. & Feng, G. S. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood 109, 862–867 (2007)
Shiroishi, M. et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl Acad. Sci. USA 100, 8856–8861 (2003)
Baldridge, M. T., King, K. Y. & Goodell, M. A. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 32, 57–65 (2011)
Ujike, A. et al. Impaired dendritic cell maturation and increased TH2 responses in PIR-B(−/−) mice. Nature Immunol. 3, 542–548 (2002)
Simsek, T. et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7, 380–390 (2010)
Zhang, C. C., Krieg, S. & Shapiro, D. J. HMG-1 stimulates estrogen response element binding by estrogen receptor from stably transfected HeLa cells. Mol. Endocrinol. 13, 632–643 (1999)
Yan, M. et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nature Med. 12, 945–949 (2006)
Zhang, C. C., Steele, A. D., Lindquist, S. & Lodish, H. F. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc. Natl Acad. Sci. USA 103, 2184–2189 (2006)
Luo, Y., Lu, Z., Raso, S. W., Entrican, C. & Tangarone, B. Dimers and multimers of monoclonal IgG1 exhibit higher in vitro binding affinities to Fcγ receptors. MAbs 1, 491–504 (2009)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011)
Acknowledgements
We thank S. Armstrong for the MSCV–MLL–AF9–IRES–YFP construct, H. Hobbs for the CMV–ANGPTL6–Flag plasmid, T. Takai for providing the PIRB knockout mice to S.-H.C., X.-J. Xie for binding analysis, and UTSW Genomics and Microarray Core facility for DNA array experiments. S.-H.C. thanks support from NIH. C.C.Z. was supported by NIH grant K01 CA 120099, American Society of Hematology Junior Faculty Award, March of Dimes Basil O’Connor Scholar Award, DOD PR093256, CPRIT RP100402, and the Gabrielle’s Angel Foundation.
Author information
Authors and Affiliations
Contributions
J.Z., M.U., C.C. and C.C.Z. were responsible for the study design, identification of receptors, binding, signalling and functional assays, data analysis and writing of the manuscript. J.L., X.C., C.Z., H.H., X.K., R.S. and X.W. were responsible for binding and signalling assays and data analysis. J.Y. and S.-H.C. carried out the ligand-binding assays, H.-Y.W. carried out AML characterization, and A.P.C. and E.S.W. carried out the SPR assay and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2 and Supplementary Figures 1-19. (PDF 2399 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Umikawa, M., Cui, C. et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 485, 656–660 (2012). https://doi.org/10.1038/nature11095
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11095
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.