Abstract
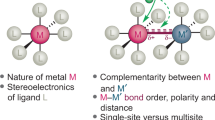
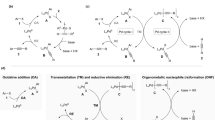
Copper and palladium catalysts are critically important in numerous commercial chemical processes. Improvements in the activity, selectivity and scope of these catalysts could drastically reduce the environmental impact, and increase the sustainability, of chemical reactions. One rapidly developing strategy for achieving these goals is to use ‘high-valent’ organometallic copper and palladium intermediates in catalysis. Here we describe recent advances involving both the fundamental chemistry and the applications of these high-valent metal complexes in numerous synthetically useful catalytic transformations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Magano, J. & Dunetz, J. R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 111, 2177–2250 (2011)
Evano, G., Blanchard, N. & Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 108, 3054–3131 (2008)
Krause, N., ed. Modern Organocopper Chemistry (Wiley-VCH, 2002)
Eckert, M., Fleischmann, G., Jira, R., Bolt, H. M. & Golka, K. in Ullmann’s Encyclopedia of Industrial Chemistry 191–207 (Wiley-VCH, 2006)
Corbet, J.-P. & Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 106, 2651–2710 (2006)
Ullmann, F. On a new formation of diphenylamine derivatives. Ber. Deutsch. Chem. Ges. 36, 2382–2384 (1903)
Ullmann, F. Over a new preparation manner of phenylethersalicylic acid. Ber. Deutsch. Chem. Ges. 37, 853–854 (1904)
Beletskaya, I. P. & Cheprakov, A. V. Copper in cross-coupling reactions: the post-Ullmann chemistry. Coord. Chem. Rev. 248, 2337–2364 (2004)
Monnier, F. & Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 48, 6954–6971 (2009)
Ribas, X. & Casitas, A. in Ideas in Chemistry and Molecular Sciences: Where Chemistry Meets Life (ed. Pignataro, B. ) 31–57 (Wiley-VCH, 2010)This is a thorough review on high-valent copper chemistry.
Sperotto, E., van Klink, G. P. M., van Koten, G. & de Vries, J. G. The mechanism of the modified Ullmann reaction. Dalton Trans. 39, 10338–10351 (2010)
Nakamura, E. & Mori, S. Wherefore art though copper? Structures and reaction mechanisms of organocuprate clusters in organic chemistry. Angew. Chem. Int. Ed. 39, 3750–3771 (2000)
Torborg, C. & Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 351, 3027–3043 (2009)
Hartwig, J. F. Electronic effects on reductive elimination to form carbon–carbon and carbon–heteroatom bonds from palladium(II) complexes. Inorg. Chem. 46, 1936–1947 (2007)
Willert-Porada, M. A., Burton, D. J. & Baenziger, N. C. Synthesis and X-ray structure of bis(trifluoromethyl)(N,N-diethyldithiocarbamato)-copper; a remarkably stable perfluoroalkylcopper(III) complex. J. Chem. Soc. Chem. Commun. 1633–1634 (1989)
Furuta, H., Maeda, H. & Osuka, A. Doubly N-confused porphyrin: a new complexing agent capable of stabilizing higher oxidation states. J. Am. Chem. Soc. 122, 803–807 (2000)
Santo, R. et al. Diamagnetic-paramagnetic conversion of tris(2-pyridylthio)methylcopper(III) through a structural change from trigonal bipyramidal to octahedral. Angew. Chem. Int. Ed. 45, 7611–7614 (2006)
Cohen, T., Wood, J. & Dietz, A. G. Organocopper intermediates in the exchange reaction of aryl halides with salts of copper(I). The possible role of copper(III). Tetrahedr. Lett. 15, 3555–3558 (1974)
Dorigo, A. E., Wanner, J. & Schleyer, P. R. Computational evidence for the existence of CuIII intermediates in addition and substitution reactions with dialkylcuprates. Angew. Chem. Int. Edn Engl. 34, 476–478 (1995)
Snyder, J. P. Mechanism of lithium cuprate conjugate addition: neutral tetracoordinate CuI cuprates as essential intermediates. J. Am. Chem. Soc. 117, 11025–11026 (1995)
Karlström, A. S. E. & Bäckvall, J.-E. Experimental evidence supporting a CuIII intermediate in cross-coupling reactions of allylic esters with diallylcuprate species. Chemistry 7, 1981–1989 (2001)
Bertz, S. H., Cope, S., Murphy, M., Ogle, C. A. & Taylor, B. J. Rapid injection NMR in mechanistic organocopper chemistry. Preparation of the elusive copper(III) intermediate. J. Am. Chem. Soc. 129, 7208–7209 (2007)This paper reports the first observation of Cu( iii ) intermediates in conjugate addition reactions using RI-NMR.
Hu, H. & Snyder, J. P. Organocuprate conjugate addition: the square-planar “CuIII” intermediate. J. Am. Chem. Soc. 129, 7210–7211 (2007)
Bertz, S. H., Cope, S., Dorton, D., Murphy, M. & Ogle, C. A. Organocuprate cross-coupling: the central role of the copper(III) intermediate and the importance of the copper(I) precursor. Angew. Chem. Int. Ed. 46, 7082–7085 (2007)
Gärtner, T., Henze, W. & Gschwind, R. M. NMR detection of Cu(III) intermediates in substitution reactions of alkyl halides with Gilman cuprates. J. Am. Chem. Soc. 129, 11362–11363 (2007)
Bartholomew, E. R., Bertz, S. H., Cope, S., Murphy, M. & Ogle, C. A. Preparation of σ- and π-allylcopper(III) intermediates in SN2 and SN2′ reactions of organocuprate(I) reagents with allylic substrates. J. Am. Chem. Soc. 130, 11244–11245 (2008)
Bertz, S. H., Miao, G. & Eriksson, M. It’s on lithium! An answer to the recent communication which asked the question: “If the cyano ligand is not on copper, then where is it? Chem. Commun. (Camb.) 815–816 (1996)
Alexakis, A., Vastra, J. & Mangeney, P. Acceleration of the conjugate addition of diethyl zinc to enones by either Cu(OTf)2 or trivalent phosphorous ligands. Tetrahedr. Lett. 38, 7745–7748 (1997)
Bartholomew, E. R. et al. Neutral organocopper(III) complexes. Chem. Commun. (Camb.) 1176–1177 (2008)
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalyzed C−H bond arylation. Science 323, 1593–1597 (2009)
Deprez, N. R. & Sanford, M. S. Synthetic and mechanistic studies of Pd-catalyzed C-H arylation with diaryliodonium salts: evidence for a bimetallic high oxidation state Pd intermediate. J. Am. Chem. Soc. 131, 11234–11241 (2009)
Chen, B., Hou, X.-L., Li, Y.-X. & Wu, Y.-D. Mechanistic understanding of the unexpected meta selectivity in copper-catalyzed anilide C−H bond arylation. J. Am. Chem. Soc. 133, 7668–7671 (2011)
Das, P., Sharma, D., Kumar, M. & Singh, B. Copper promoted C−N and C−O type cross-coupling reactions. Curr. Org. Chem. 14, 754–783 (2010)
Chan, D. M. T. & Lam, P. Y. S. in Boronic Acids (ed. Hall, D. G. ) 205–240 (Wiley-VCH, 2005)
Casitas, A. et al. Direct observation of CuI/CuIII redox steps relevant to Ullmann-type coupling reactions. Chem. Sci. 1, 326–330 (2010)
Kunz, K., Scholz, U. & Ganzer, D. Renaissance of Ullmann and Goldberg reactions: progress in copper catalyzed C−N-, C−O-, and C−S-coupling. Synlett 15, 2428–2439 (2003)
King, A. E. et al. Copper-catalyzed aerobic oxidative functionalization of an arene C−H bond: evidence for an aryl-copper(III) intermediate. J. Am. Chem. Soc. 132, 12068–12073 (2010)This paper demonstrates a Cu( iii )–aryl complex to be a catalytically relevant intermediate in a carbon–hydrogen bond amination and methoxylation reaction.
Brasche, G. & Buchwald, S. L. C–H functionalization/C–N bond formation: copper-catalyzed synthesis of benzimidazoles from amidines. Angew. Chem. Int. Ed. 47, 1932–1934 (2008)
Ueda, S. & Nagasawa, H. Synthesis of 2-arylbenzoxazoles by copper-catalyzed intramolecular oxidative C–O coupling of benzanilides. Angew. Chem. Int. Ed. 47, 6411–6413 (2008)
Hamada, T., Ye, X. & Stahl, S. S. Copper-catalyzed aerobic oxidative amidation of terminal alkynes: efficient synthesis of ynamides. J. Am. Chem. Soc. 130, 833–835 (2008)
Uemura, T., Imoto, S. & Chatani, N. Amination of the ortho C–H bonds by the Cu(OAc)2-mediated reaction of 2-phenylpyridines with anilines. Chem. Lett. 35, 842–843 (2006)
Mizuhara, T., Inuki, S., Oishi, S., Fujii, N. & Ohno, H. Cu(II)-mediated oxidative intermolecular ortho C−H functionalisation using tetrahydropyrimidine as the directing group. Chem. Commun. (Camb.) 3413–3415 (2009)
Huffman, L. M. & Stahl, S. S. Carbon−nitrogen bond formation involving well-defined aryl-copper(III) complexes. J. Am. Chem. Soc. 130, 9196–9197 (2008)
Tye, J. W., Weng, Z., Johns, A. M., Incarvito, C. D. & Hartwig, J. F. Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides. J. Am. Chem. Soc. 130, 9971–9983 (2008)
Jones, G. O., Liu, P., Houk, K. N. & Buchwald, S. L. Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions. J. Am. Chem. Soc. 132, 6205–6213 (2010)
Yu, H.-Z., Jiang, Y.-Y., Fu, Y. & Liu, L. Alternative mechanistic explanation for ligand-dependent selectivities in copper-catalyzed N- and O-arylation reactions. J. Am. Chem. Soc. 132, 18078–18091 (2010)
Ribas, X. et al. Aryl C−H activation by CuII to form an organometallic aryl-CuIII species: a novel twist on copper disproportionation. Angew. Chem. Int. Ed. 41, 2991–2994 (2002)This paper reports an early example of an isolatable, well-characterized Cu( iii )–aryl complex.
Wendlandt, A. E., Suess, A. M. & Stahl, S. S. Copper-catalyzed aerobic oxidative C–H functionalizations: trends and mechanistic insights. Angew. Chem. Int. Ed. 50, 11062–11087 (2011)
Ueda, S. & Nagasawa, H. Copper-catalyzed synthesis of benzoxazoles via a regioselective C-H functionalization/C-O bond formation under an air atmosphere. J. Org. Chem. 74, 4272–4277 (2009)
Siemsen, P., Livingston, R. C. & Diederich, F. Acetylenic coupling: a powerful tool in molecular construction. Angew. Chem. Int. Ed. 39, 2632–2657 (2000)
Gao, Y. et al. Copper-catalyzed aerobic oxidative coupling of terminal alkynes with H-phosphates leading to alkynylphosphonates. J. Am. Chem. Soc. 131, 7956–7957 (2009)
Chen, X., Hao, X.-S., Goodhue, C. E. & Yu, J.-Q. Cu(II)-catalyzed functionalizations of aryl C−H bonds using O2 as an oxidant. J. Am. Chem. Soc. 128, 6790–6791 (2006)
Wang, W., Luo, F., Zhang, S. & Cheng, J. Copper(II)-catalyzed ortho-acyloxylation of the 2-arylpyridines sp2 C−H bonds with anhydrides, using O2 as terminal oxidant. J. Org. Chem. 75, 2415–2418 (2010)
Casitas, A., Canta, M., Solá, M., Costas, M. & Ribas, X. Nucleophilic aryl fluorination and aryl halide exchange mediated by a CuI/CuIII catalytic cycle. J. Am. Chem. Soc. 133, 19386–19392 (2011)
Heck, R. F. Aromatic haloethylation with palladium and copper halides. J. Am. Chem. Soc. 90, 5538–5542 (1968)
Fahey, D. R. The coordination-catalyzed ortho-halogenation of azobenzene. J. Organomet. Chem. 27, 283–292 (1971)
Tremont, S. J. & Rahman, H. U. Ortho-alkylation of acetanilides using alkyl halides and palladium acetate. J. Am. Chem. Soc. 106, 5759–5760 (1984)
Byers, P. K., Canty, A. J., Skelton, B. W. & White, A. H. Oxidative addition of iodomethane to [PdMe2(bpy)] and the X-ray structure of the organopalladium(iv) product fac-[PdMe3(bpy)I] (bpy = 2,2′bipyridyl). J. Chem. Soc. Chem. Commun.1722–1724 (1986)
Cotton, F. A. et al. High yield syntheses of stable, singly bonded Pd26+ compounds. J. Am. Chem. Soc. 128, 13674–13675 (2006)
Roy, A. H. & Hartwig, J. F. Directly observed reductive elimination of aryl halides from monomeric arylpalladium(II) halide complexes. J. Am. Chem. Soc. 125, 13944–13945 (2003)
Collman, J. P., Hegedus, L. S., Norton, J. R. & Finke, R. G. Principles and Applications of Organotransition Metal Chemistry 2nd edn 322–333 (University Science Books, 1982)
Canty, A. J. Organopalladium and platinum chemistry in oxidizing milieu as models for organic synthesis involving the higher oxidation states of palladium. Dalton Trans. 47, 10409–10417 (2009)
Muñiz, K. High-oxidation-state palladium catalysis: new reactivity for organic synthesis. Angew. Chem. Int. Ed. 48, 9412–9423 (2009)This is a thorough review on high-valent palladium chemistry.
Xu, L.-M., Li, B.-J., Yang, Z. & Shi, Z.-J. Organopalladium(IV) chemistry. Chem. Soc. Rev. 39, 712–733 (2010)
Vicente, J., Arcas, A., Julia-Hernandez, F. & Bautista, D. Synthesis, isolation, and characterization of an organometallic triiodopalladium(IV) complex. Quantitative and regioselective synthesis of two C–I reductive elimination products. Inorg. Chem. 50, 5339–5341 (2011)
Arnold, P. L., Sanford, M. S. & Pearson, S. M. Chelating N-heterocyclic carbene alkoxide as a supporting ligand for PdII/IV C-H bond functionalization catalysis. J. Am. Chem. Soc. 131, 13912–13913 (2009)
Ball, N. D. & Sanford, M. S. Synthesis and reactivity of a mono-σ-aryl palladium(IV) fluoride complex. J. Am. Chem. Soc. 131, 3796–3797 (2009)
Alsters, P. L. et al. Rigid five- and six-membered C,N,N′-bound aryl, benzyl, and alkyl organopalladium complexes: sp2 vs sp3 C-H activation during cyclopalladation and palladium(IV) intermediates in oxidative addition reactions with dihalogens and alkyl halides. Organometallics 12, 1831–1844 (1993)
Whitfield, S. R. & Sanford, M. S. Reactivity of Pd(II) complexes with electrophilic chlorinating reagents: isolation of Pd(IV) products and observation of C–Cl bond-forming reductive elimination. J. Am. Chem. Soc. 129, 15142–15143 (2007)
Furuya, T. et al. Mechanism of C–F reductive elimination from palladium(IV) fluoride. J. Am. Chem. Soc. 132, 3793–3807 (2010)
Powers, D. C., Xiao, D. Y., Geibel, M. A. L. & Ritter, T. On the mechanism of palladium-catalyzed aromatic C–H oxidation. J. Am. Chem. Soc. 132, 14530–14536 (2010)This paper describes mechanistic studies implicating a Pd( iii ) dimer intermediate in a palladium-catalysed carbon–hydrogen chlorination reaction described in ref. 85.
Sehnal, P., Taylor, R. J. K. & Fairlamb, I. J. S. Emergence of palladium(IV) chemistry in synthesis and catalysis. Chem. Rev. 110, 824–889 (2010)
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010)This is a comprehensive review of palladium-catalysed, ligand-directed carbon–hydrogen functionalization reactions.
Wu, T., Yin, G. & Liu, G. Palladium-catalyzed intramolecular aminofluorination of unactivated alkenes. J. Am. Chem. Soc. 131, 16354–16355 (2009)
Michael, F. E., Sibbald, P. A. & Cochran, B. M. Palladium-catalyzed intramolecular chloroamination of alkenes. Org. Lett. 10, 793–796 (2008)
Kalyani, D., Satterfield, A. D. & Sanford, M. S. Palladium-catalyzed oxidative arylhalogenation of alkenes: synthetic scope and mechanistic insights. J. Am. Chem. Soc. 132, 8419–8427 (2010)This paper describes high-valent palladium-catalysed arylhalogenation reactions of α-alkenes and demonstrates complementarity to low-valent methods.
Oestreich, M., ed. The Mizoroki-Heck Reaction (Wiley, 2009)
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007)
Grushin, V. V. The organometallic fluorine chemistry of palladium and rhodium: studies toward aromatic fluorination. Acc. Chem. Res. 43, 160–171 (2010)
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010)
Ball, N. D., Gary, J. B., Ye, Y. & Sanford, M. S. Mechanistic and computational studies of oxidatively-induced bond-formation at Pd: rational design of room temperature trifluoromethylation. J. Am. Chem. Soc. 133, 7577–7584 (2011)
Wang, X., Truesdale, L. & Yu, J.-Q. Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter. J. Am. Chem. Soc. 132, 3648–3649 (2010)This paper reports a novel carbon–hydrogen trifluoromethylation reaction catalysed by putative high-valent palladium.
Ye, Y., Ball, N. D., Kampf, J. W. & Sanford, M. S. Oxidation of catalytically relevant palladium dimer with “CF3+”: formation and reactivity of a monomeric palladium(IV) aquo product. J. Am. Chem. Soc. 132, 14682–14687 (2010)This paper provides evidence supporting the possibility of a high-valent Pd( iv ) intermediate in the carbon–hydrogen trifluoromethylation reaction described in ref. 82.
Powers, D. C., Geibel, M. A. L., Klein, J. E. M. N. & Ritter, T. Bimetallic palladium catalysis: direct observation of Pd(III)-Pd(III) intermediates. J. Am. Chem. Soc. 131, 17050–17051 (2009)
Dick, A. R., Hull, K. L. & Sanford, M. S. A highly selective catalytic method for the oxidative functionalization of C-H bonds. J. Am. Chem. Soc. 126, 2300–2301 (2004)
Xu, J. et al. Copper-catalyzed trifluoromethylation of aryl boronic acids using a CF3+ reagent. Chem. Commun. (Camb.) 47, 4300–4302 (2011)
Deprez, N. R., Kalyani, D., Krause, A. & Sanford, M. S. Room temperature palladium-catalyzed 2-arylation of indoles. J. Am. Chem. Soc. 128, 4972–4973 (2006)
Phipps, R. J., Grimster, N. P. & Gaunt, M. J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 130, 8172–8174 (2008)
Zhang, J., Khaskin, E., Anderson, N. P., Zavalij, P. Y. & Vedernikov, A. N. Catalytic aerobic oxidation of substituted 8-methylquinolines in PdII-2,6-pyridine dicarboxylic acid systems. Chem. Commun. (Camb.) 3625–3627 (2008)
Zhang, Y.-H. & Yu, J.-Q. Pd(II)-catalyzed hydroxylation of arenes with 1 atm O2 or air. J. Am. Chem. Soc. 131, 14654–14655 (2009)
Hull, K. L., Lanni, E. L. & Sanford, M. S. Highly regioselective catalytic oxidative coupling reactions: synthetic scope and mechanistic insights. J. Am. Chem. Soc. 128, 14047–14049 (2006)
Rosewall, C. F., Sibbald, P. A., Liskin, D. V. & Michael, F. E. Palladium-catalyzed carboamination of alkenes promoted by N-fluorobenzenesulfonimide via C–H activation of arenes. J. Am. Chem. Soc. 131, 9488–9489 (2009)
Hickman, A. J. &. Sanford, M. S. Catalyst control of site selectivity in the PdII/IV-catalyzed direct arylation of naphthalene. ACS Catal. 1, 170–174 (2011)
Racowski, J. M., Ball, N. D. & Sanford, M. S. C–H bond activation at palladium(IV) centers. J. Am. Chem. Soc. 133, 18022–18025 (2011)
Grove, D. M., van Koten, F., Zoet, R., Murrall, N. W. & Welch, A. J. Unique stable organometallic nickel(III) complexes; syntheses and the molecular structure of [Ni[C6H3(CH2NMe2)2-2,6]I2]. J. Am. Chem. Soc. 105, 1379–1380 (1983)
Ceder, R. M., Granell, J. & Muller, G. Preparation of five-membered nickelacycles with anionic C-N-N′ terdentate ligands. X-ray crystal structure of [NiCl{2-(CH = NCH2CH2NMe2)-3-ClC6H3}]. Organometallics 15, 4618–4624 (1996)
Higgs, A. T., Zinn, P. J., Simmons, S. J. & Sanford, M. S. Oxidatively induced carbon-halogen bond-forming reactions at nickel. Organometallics 28, 6142–6144 (2009)
Muñiz, K. & Streuff, J. Exploring the nickel-catalyzed oxidation of alkenes: a diamination by sulfanamide transfer. Angew. Chem. Int. Ed. 46, 7125–7127 (2007)
Lin, B. L., Clough, C. R. & Hillhouse, G. L. Interactions of aziridines with nickel complexes: oxidative-addition and reductive-elimination reactions that break and make C−N bonds. J. Am. Chem. Soc. 124, 2890–2891 (2002)
Tang, P. & Furuya, T. &. Ritter, T. Silver-catalyzed late-stage fluorination. J. Am. Chem. Soc. 132, 12150–12154 (2010)
Acknowledgements
The work of the group of M.S.S. described herein was supported by the NIH-NIGMS (GM073836) and by the US NSF (CHE-0545909 and CHE-1111563).
Author information
Authors and Affiliations
Contributions
A.J.H. and M.S.S. worked together to outline the content, as well as write and edit the manuscript, references and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hickman, A., Sanford, M. High-valent organometallic copper and palladium in catalysis. Nature 484, 177–185 (2012). https://doi.org/10.1038/nature11008
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11008
This article is cited by
-
Asymmetric-waveform alternating current-promoted silver catalysis for C–H phosphorylation
Nature Synthesis (2023)
-
Catalytic remote hydrohalogenation of internal alkenes
Nature Chemistry (2022)
-
Copper(II) phthalocyanine as an efficient and versatile catalyst for click reactions at room temperature
Journal of the Iranian Chemical Society (2022)
-
Suzuki cross-coupling reactions over engineered AuPd alloy nanoparticles by recycling scattered light
Nano Research (2022)
-
Heterogeneous Cu catalyst in organic transformations
Nano Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.