Abstract
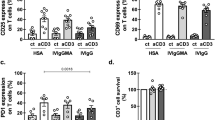
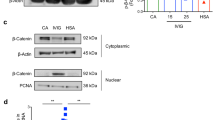
High-dose intravenous immunoglobulin is a widely used therapeutic preparation of highly purified immunoglobulin G (IgG) antibodies. It is administered at high doses (1–2 grams per kilogram) for the suppression of autoantibody-triggered inflammation in a variety of clinical settings1. This anti-inflammatory activity of intravenous immunoglobulin is triggered by a minor population of IgG crystallizable fragments (Fcs), with glycans terminating in α2,6 sialic acids (sFc) that target myeloid regulatory cells expressing the lectin dendritic-cell-specific ICAM-3 grabbing non-integrin (DC-SIGN; also known as CD209)2,3,4. Here, to characterize this response in detail, we generated humanized DC-SIGN mice (hDC-SIGN), and demonstrate that the anti-inflammatory activity of intravenous immunoglobulin can be recapitulated by the transfer of bone-marrow-derived sFc-treated hDC-SIGN+ macrophages or dendritic cells into naive recipients. Furthermore, sFc administration results in the production of IL-33, which, in turn, induces expansion of IL-4-producing basophils that promote increased expression of the inhibitory Fc receptor FcγRIIB on effector macrophages. Systemic administration of the TH2 cytokines IL-33 or IL-4 upregulates FcγRIIB on macrophages, and suppresses serum-induced arthritis. Consistent with these results, transfer of IL-33-treated basophils suppressed induced arthritic inflammation. This novel DC-SIGN–TH2 pathway initiated by an endogenous ligand, sFc, provides an intrinsic mechanism for maintaining immune homeostasis that could be manipulated to provide therapeutic benefit in autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
06 July 2011
Fig. 2b and d were corrected.
References
Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory actions of intravenous immunoglobulin. Annu. Rev. Immunol. 26, 513–533 (2008)
Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006)
Anthony, R. M. et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 (2008)
Anthony, R. M., Wermeling, F., Karlsson, M. C. & Ravetch, J. V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl Acad. Sci. USA 105, 19571–19578 (2008)
Granelli-Piperno, A. et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175, 4265–4273 (2005)
Soilleux, E. J. et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro . J. Leukoc. Biol. 71, 445–457 (2002)
Geijtenbeek, T. B. & Gringhuis, S. I. Signalling through C-type lectin receptors: shaping immune responses. Nature Rev. Immunol. 9, 465–479 (2009)
Gringhuis, S. I. et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 26, 605–616 (2007)
Hodges, A. et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nature Immunol. 8, 569–577 (2007)
Korganow, A. S. et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 10, 451–461 (1999)
Schaefer, M. et al. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J. Immunol. 180, 6836–6845 (2008)
Pricop, L. et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 166, 531–537 (2001)
Bruhns, P., Samuelsson, A., Pollard, J. W. & Ravetch, J. V. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity 18, 573–581 (2003)
Samuelsson, A., Towers, T. L. & Ravetch, J. V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291, 484–486 (2001)
Finkelman, F. D. et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151, 1235–1244 (1993)
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005)
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010)
Paul, W. E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nature Rev. Immunol. 10, 225–235 (2010)
Saenz, S. A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010)
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo . Immunity 15, 985–995 (2001)
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 3, 673–680 (2002)
Wang, Y. H. et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 204, 1837–1847 (2007)
Min, B. et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 200, 507–517 (2004)
Seder, R. A. et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc. Natl Acad. Sci. USA 88, 2835–2839 (1991)
Seder, R. A. et al. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int. Arch. Allergy Appl. Immunol. 94, 137–140 (1991)
Wang, M., Saxon, A. & Diaz-Sanchez, D. Early IL-4 production driving Th2 differentiation in a human in vivo allergic model is mast cell derived. Clin. Immunol. 90, 47–54 (1999)
Shinkai, K., Mohrs, M. & Locksley, R. M. Helper T cells regulate type-2 innate immunity in vivo . Nature 420, 825–829 (2002)
Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 9, 310–318 (2008)
Mohrs, M., Shinkai, K., Mohrs, K. & Locksely, R. M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001)
Ohmura, K et al. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 52, 1866–1875 (2005)
Xu, D. et al. IL-33 exacerbates autoantibody-induced arthritis. J. Immunol. 184, 2620–2626 (2010)
Scherer, H. U. et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 62, 1620–1629 (2010)
van de Geijn, F. E. et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11, R193 (2009)
Takai, T., Ono, M., Hikida, M., Ohmori, H. & Ravetch, J. V. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature 379, 346–349 (1996)
Lanoue, A. et al. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J. Exp. Med. 200, 1383–1393 (2004)
Obata, K. et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 (2007)
Cheong, C. et al. New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells. J. Immunol. Methods 360, 66–75 (2010)
Finkelman, F., Morris, S., Orekhova, T. & Sehy, D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr. Protoc. Immunol. 6.28.1–6.28.10. (2003)
Jeffrey, K. L. et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nature Immunol. 7, 274–283 (2006)
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992)
Ohmori, K. et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J. Immunol. 182, 2835–2841 (2009)
Acknowledgements
The authors thank P. Smith, M. Kibe, J. Brown and K. Velinzon for technical support, K. L. Jeffrey, C. Cheong, R. Steinman and J. J. Lee for discussions, M. Pack for providing human spleen sections, A. McKenzie for providing SIGN-R1−/− mice, T. Sparwasser for providing CD11c-hDC-SIGN mice, C. G. Park for providing hDC-SIGN and hDC-SIGN-R expressing cell lines, and H. Watarai for providing anti-IL-25R antibodies. R.M.A. is an Irvington Institute fellow of the Cancer Research Institute. F.W. is supported by the Wenner-Gren Foundations, Sweden. This work was performed with support from Virdante Pharmaceuticals and NIH grants to J.V.R.
Author information
Authors and Affiliations
Contributions
R.M.A., T.K., F.W. and J.V.R. designed the experiments and interpreted the results. R.M.A., T.K. and F.W. performed the experiments, and R.M.A. and J.V.R. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-12 with legends. (PDF 1114 kb)
Rights and permissions
About this article
Cite this article
Anthony, R., Kobayashi, T., Wermeling, F. et al. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 475, 110–113 (2011). https://doi.org/10.1038/nature10134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10134
This article is cited by
-
Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection
Cellular & Molecular Immunology (2023)
-
Glycobiology of rheumatic diseases
Nature Reviews Rheumatology (2023)
-
Production of recombinant human IgG1 Fc with beneficial N-glycosylation pattern for anti-inflammatory activity using genome-edited chickens
Communications Biology (2023)
-
Control of lupus activity during pregnancy via the engagement of IgG sialylation: novel crosstalk between IgG sialylation and pDC functions
Frontiers of Medicine (2023)
-
Aberrant Immunoglobulin G Glycosylation in Multiple Sclerosis
Journal of Neuroimmune Pharmacology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.