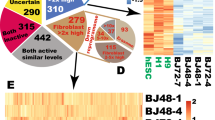
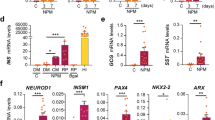
Abstract
As is the case for embryo-derived stem cells, application of reprogrammed human induced pluripotent stem cells is limited by our understanding of lineage specification. Here we demonstrate the ability to generate progenitors and mature cells of the haematopoietic fate directly from human dermal fibroblasts without establishing pluripotency. Ectopic expression of OCT4 (also called POU5F1)-activated haematopoietic transcription factors, together with specific cytokine treatment, allowed generation of cells expressing the pan-leukocyte marker CD45. These unique fibroblast-derived cells gave rise to granulocytic, monocytic, megakaryocytic and erythroid lineages, and demonstrated in vivo engraftment capacity. We note that adult haematopoietic programs are activated, consistent with bypassing the pluripotent state to generate blood fate: this is distinct from haematopoiesis involving pluripotent stem cells, where embryonic programs are activated. These findings demonstrate restoration of multipotency from human fibroblasts, and suggest an alternative approach to cellular reprogramming for autologous cell-replacement therapies that avoids complications associated with the use of human pluripotent stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
24 July 2018
In this Article, there were duplicated empty lanes in Supplementary Figs. 2e and 3b. The corrected figures are presented in the Supplementary Information to the accompanying Amendment. The original Article has not been corrected.
References
Jaenisch, R. & Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 (2008)
Chan, E. M. et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nature Biotechnol. 27, 1033–1037 (2009)
Lin, T. et al. A chemical platform for improved induction of human iPSCs. Nature Methods 6, 805–808 (2009)
Mikkelsen, T. S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008)
Kanawaty, A. & Henderson, J. Genomic analysis of induced pluripotent stem (iPS) cells: routes to reprogramming. Bioessays 31, 134–138 (2009)
Feng, R. et al. PU.1 and C/EBPα/β convert fibroblasts into macrophage-like cells. Proc. Natl Acad. Sci. USA 105, 6057–6062 (2008)
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010)
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010)
Kang, J., Shakya, A. & Tantin, D. Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem. Sci. 34, 491–499 (2009)
Brunner, C. et al. B cell-specific transgenic expression of Bcl2 rescues early B lymphopoiesis but not B cell responses in BOB.1/OBF.1-deficient mice. J. Exp. Med. 197, 1205–1211 (2003)
Emslie, D. et al. Oct2 enhances antibody-secreting cell differentiation through regulation of IL-5 receptor α chain expression on activated B cells. J. Exp. Med. 205, 409–421 (2008)
Pfisterer, P. et al. CRISP-3, a protein with homology to plant defense proteins, is expressed in mouse B cells under the control of Oct2. Mol. Cell. Biol. 16, 6160–6168 (1996)
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006)
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)
Hassan, H. T. & Zander, A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol. 95, 257–262 (1996)
Lyman, S. D. et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75, 1157–1167 (1993)
Kim, J. B. et al. Direct reprogramming of human neural stem cells by OCT4. Nature 461, 649–653 (2009)
Lebofsky, R. & Walter, J. C. New Myc-anisms for DNA replication and tumorigenesis? Cancer Cell 12, 102–103 (2007)
Silverstein, S. C., Steinman, R. M. & Cohn, Z. A. Endocytosis. Annu. Rev. Biochem. 46, 669–722 (1977)
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 5, 738–743 (2004)
Roy, N. S. et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature Med. 12, 1259–1268 (2006)
Amariglio, N. et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, e1000029 (2009)
Fried, W. Erythropoietin and erythropoiesis. Exp. Hematol. 37, 1007–1015 (2009)
Perlingeiro, R. C., Kyba, M. & Daley, G. Q. Clonal analysis of differentiating embryonic stem cells reveals a hematopoietic progenitor with primitive erythroid and adult lymphoid-myeloid potential. Development 128, 4597–4604 (2001)
Debili, N. et al. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood 88, 1284–1296 (1996)
Klimchenko, O. et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 114, 1506–1517 (2009)
Strodtbeck, D. et al. Graft clonogenicity and intensity of pre-treatment: factors affecting outcome of autologous peripheral hematopoietic cell transplantation in patients with acute myeloid leukemia in first remission. Bone Marrow Transplant. 36, 1083–1088 (2005)
Orkin, S. H. & Zon, L. I. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nature Immunol. 3, 323–328 (2002)
Shivdasani, R. A., Mayer, E. L. & Orkin, S. H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373, 432–434 (1995)
Ichikawa, M., Asai, T., Chiba, S., Kurokawa, M. & Ogawa, S. Runx1/AML-1 ranks as a master regulator of adult hematopoiesis. Cell Cycle 3, 722–724 (2004)
Friedman, A. D. Transcriptional control of granulocyte and monocyte development. Oncogene 26, 6816–6828 (2007)
Koschmieder, S., Rosenbauer, F., Steidl, U., Owens, B. M. & Tenen, D. G. Role of transcription factors C/EBPα and PU.1 in normal hematopoiesis and leukemia. Int. J. Hematol. 81, 368–377 (2005)
Ng, E. S. et al. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development 132, 873–884 (2005)
Tsai, F. Y. et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–226 (1994)
Vijayaragavan, K. et al. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell 4, 248–262 (2009)
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005)
Kistler, B., Pfisterer, P. & Wirth, T. Lymphoid- and myeloid-specific activity of the PU.1 promoter is determined by the combinatorial action of octamer and ets transcription factors. Oncogene 11, 1095–1106 (1995)
Rodda, D. J. et al. Transcriptional regulation of nanog by OCT4 and SOX2. J. Biol. Chem. 280, 24731–24737 (2005)
Sridharan, R. et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell 136, 364–377 (2009)
Ghozi, M. C., Bernstein, Y., Negreanu, V., Levanon, D. & Groner, Y. Expression of the human acute myeloid leukemia gene AML1 is regulated by two promoter regions. Proc. Natl Acad. Sci. USA 93, 1935–1940 (1996)
Chang, K. H. et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood 108, 1515–1523 (2006)
Kwon, U. K., Yen, P. H., Collins, T. & Wells, R. A. Differential lineage-specific regulation of murine CD45 transcription by Oct-1 and PU.1. Biochem. Biophys. Res. Commun. 344, 146–154 (2006)
Feugier, P. et al. Hematologic recovery after autologous PBPC transplantation: importance of the number of postthaw CD34+ cells. Transfusion 43, 878–884 (2003)
Rampalli, S. et al. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nature Struct. Mol. Biol. 14, 1150–1156 (2007)
Oshima, A. et al. Cloning, sequencing, and expression of cDNA for human β-glucuronidase. Proc. Natl Acad. Sci. USA 84, 685–689 (1987)
Li, C. & Wong, W. H. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl Acad. Sci. USA 98, 31–36 (2001)
Acknowledgements
This work was supported by grants to M.B. from the Canadian Institute of Health Research (CIHR), the Canadian Cancer Society Research Institute (CCS-RI), the StemCell Network and the Ontario Ministry of Research Innovation (MRI). M.B. is supported by the Canadian Chair Program and holds the Canada Research Chair in human stem cell biology. E.S. is supported by Ministry of Research and Innovation (MRI) and MITACS fellowships, R.M.R is supported by a CCS-RI fellowship and R.M. is supported by an Ontario Graduate Scholarship (OGS). We thank T. Werbowetski-Ogilvie for her help.
Author information
Authors and Affiliations
Contributions
All authors contributed to the acquisition, analysis and interpretation of the data; E.S., S.R., R.M.R. and M.B. initiated and designed the study; A.S. performed Affymetrix analyses; R.M.R. performed in vivo analyses; E.S., S.R., R.M.R. and M.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Tables
This file contains Supplementary Tables 1 - 4. (PDF 897 kb)
Supplementary Figures
This file contains Supplementary Figures 1-18 with legends and additional references. (PDF 19780 kb)
Rights and permissions
About this article
Cite this article
Szabo, E., Rampalli, S., Risueño, R. et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468, 521–526 (2010). https://doi.org/10.1038/nature09591
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09591
This article is cited by
-
Kardiale Entwicklungs- und Regenerationsmechanismen
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2023)
-
Cellular direct conversion by cell penetrable OCT4-30Kc19 protein and BMP4 growth factor
Biomaterials Research (2022)
-
Pioneer factors as master regulators of the epigenome and cell fate
Nature Reviews Molecular Cell Biology (2022)
-
Transient nuclear deformation primes epigenetic state and promotes cell reprogramming
Nature Materials (2022)
-
Directly reprogrammed natural killer cells for cancer immunotherapy
Nature Biomedical Engineering (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.