Abstract
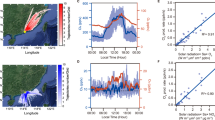
Halogen atoms and oxides are highly reactive and can profoundly affect atmospheric composition. Chlorine atoms can decrease the lifetimes of gaseous elemental mercury1 and hydrocarbons such as the greenhouse gas methane2. Chlorine atoms also influence cycles that catalytically destroy or produce tropospheric ozone3, a greenhouse gas potentially toxic to plant and animal life. Conversion of inorganic chloride into gaseous chlorine atom precursors within the troposphere is generally considered a coastal or marine air phenomenon4. Here we report mid-continental observations of the chlorine atom precursor nitryl chloride at a distance of 1,400 km from the nearest coastline. We observe persistent and significant nitryl chloride production relative to the consumption of its nitrogen oxide precursors. Comparison of these findings to model predictions based on aerosol and precipitation composition data from long-term monitoring networks suggests nitryl chloride production in the contiguous USA alone is at a level similar to previous global estimates for coastal and marine regions5. We also suggest that a significant fraction of tropospheric chlorine atoms6 may arise directly from anthropogenic pollutants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



 over the US.
over the US.

Similar content being viewed by others
References
Donohoue, D. L., Bauer, D. & Hynes, A. J. Temperature and pressure dependent rate coefficients for the reaction of Hg with Cl and the reaction of Cl with Cl: a pulsed laser photolysis-pulsed laser induced fluorescence study. J. Phys. Chem. A 109, 7732–7741 (2005)
Platt, U., Allan, W. & Lowe, D. Hemispheric average Cl atom concentration from 13C/12C ratios in atmospheric methane. Atmos. Chem. Phys. 4, 2393–2399 (2004)
Knipping, E. M. & Dabdub, D. Impact of chlorine emissions from sea-salt aerosol on coastal urban ozone. Environ. Sci. Technol. 37, 275–284 (2003)
Von Glasow, R. & Crutzen, P. J. in The Atmosphere (ed. Keeling, R. F.) 21–64 (Oxford Univ. Press, 2007)
Osthoff, H. D. et al. High levels of nitryl chloride in the polluted subtropical marine boundary layer. Nature Geosci. 1, 324–328 (2008)
Allan, W., Struthers, H. & Lowe, D. C. Methane carbon isotope effects caused by atomic chlorine in the marine boundary layer: global model results compared with Southern Hemisphere measurements. J. Geophys. Res. D 112 D04306 10.1029/2006JD007369 (2007)
Finlayson-Pitts, B. J., Ezell, M. J. & Pitts, J. N. Formation of chemically active chlorine compounds by reactions of atmospheric NaCl particles with gaseous N2O5 and ClONO2 . Nature 337, 241–244 (1989)
Behnke, W., George, C., Scheer, V. & Zetzsch, C. Production and decay of ClNO2 from the reaction of gaseous N2O5 with NaCl solution: bulk and aerosol experiments. J. Geophys. Res. D 102, 3795–3804 (1997)
Graedel, T. E. & Keene, W. C. Tropospheric budget of reactive chlorine. Glob. Biogeochem. Cycles 9, 47–77 (1995)
Kercher, J., Riedel, T. & Thornton, J. Chlorine activation by N2O5: simultaneous, in situ detection of ClNO2 and N2O5 by chemical ionization mass spectrometry. Atmos. Meas. Techn. 2, 193–204 (2009)
Pechtl, S. & von Glasow, R. Reactive chlorine in the marine boundary layer in the outflow of polluted continental air: a model study. Geophys. Res. Lett. 34 L11813 10.1029/2007GL02976 (2007)
Logan, J. A., Prather, M. J., Wofsy, S. C. & McElroy, M. B. Tropospheric chemistry: a global perspective. J. Geophys. Res. 86, 7210–7254 (1981)
Alexander, B. et al. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 9, 5043–5056 (2009)
Nelson, H. H. & Johnston, H. S. Kinetics of the reaction of Cl with ClNO and ClNO2 and the photochemistry of ClNO2 . J. Phys. Chem. 85, 3891–3896 (1981)
Abuduwailli, J., Gabchenko, M. V. & Xu, J. R. Eolian transport of salts — a case study in the area of Lake Ebinur (Xinjiang, Northwest China). J. Arid Environ. 72, 1843–1852 (2008)
Thornton, J. A., Braban, C. F. & Abbatt, J. P. D. N2O5 hydrolysis on sub-micron organic aerosols: the effect of relative humidity, particle phase, and particle size. Phys. Chem. Chem. Phys. 5, 4593–4603 (2003)
Wexler, A. S. & Clegg, S. L. Atmospheric aerosol models for systems including the ions H+, NH4 +, Na+, SO4 2-, NO3 -,Cl-, Br-, and H2O. J. Geophys. Res. D 107 4207 10.1029/2001JD000451 (2002)
Roberts, J. M., Osthoff, H. D., Brown, S. S. & Ravishankara, A. R. Laboratory measurements of ClNO2 yields from N2O5 reactions as a function of chloride molarity in various inorganic salt solutions. Geophys. Res. Lett. 36 L20808 10.1029/2009GL040448 (2009)
Moffet, R. C., de Foy, B., Molina, L. T., Molina, M. J. & Prather, K. A. Measurement of ambient aerosols in northern Mexico City by single particle mass spectrometry. Atmos. Chem. Phys. 8, 4499–4516 (2008)
Keene, W. C. & Savoie, D. L. The pH of deliquesced sea-salt aerosol in polluted marine air. Geophys. Res. Lett. 25, 2181–2184 (1998)
Olivier, J. G. J. & Berdowski, J. J. M. in The Climate System (eds Berdowski, J., Guicherit, R. & Heij, B. J.) 33–78 (Balkema, and Swets and Zeitlinger, Lisse, The Netherlands, 2001)
National Atmospheric Deposition Program Office. National Atmospheric Deposition Program (NRSP-3) (Illinois State Water Survey, Champaign, Illinois, 2010)
Malm, W. C., Sisler, J. F., Huffman, D., Eldred, R. A. & Cahill, T. A. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J. Geophys. Res. D 99, 1347–1370 (1994)
Erickson, D. J., Seuzaret, C., Keene, W. C. & Gong, S. L. A general circulation model based calculation of HCl and ClNO2 production from sea salt dechlorination: reactive chlorine emissions inventory. J. Geophys. Res. D 104, 8347–8372 (1999)
Zhang, Q. et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 34 L13801 10.1029/2007GL029979 (2007)
Bertram, T. H. et al. Direct measurements of N2O5 reactivity on ambient aerosol particles. Geophys. Res. Lett. 36 L19803 10.1029/2009GL040248 (2009)
Brown, S. S. et al. Variability in nocturnal nitrogen oxide processing and its role in regional air quality. Science 311, 67–70 (2006)
Singh, H. B. & Hanst, P. L. Peroxyacetyl nitrate (PAN) in the unpolluted atmosphere — an important reservoir for nitrogen oxides. Geophys. Res. Lett. 8, 941–944 (1981)
Grannas, A. M. et al. An overview of snow photochemistry: evidence, mechanisms and impacts. Atmos. Chem. Phys. 7, 4329–4373 (2007)
Shindell, D. T. et al. Improved attribution of climate forcing to emissions. Science 326, 716–718 (2009)
Acknowledgements
Funding for the ClNO2 observations and analysis was provided by NSF grants ATM-0633897 and ATM-0846183 to J.A.T. Boulder field measurements were supported in part by the NOAA Atmospheric Chemistry and Climate Program. J.P.K. thanks the Camille and Henry Dreyfus Foundation for a postdoctoral fellowship in environmental chemistry. N.L.W. thanks the National Research Council for a postdoctoral fellowship.
Author Contributions This was largely a collaborative effort. All authors contributed to the collection of observations during the 2009 intensive campaign described here. J.P.K., W.P.D., J.A.T. and S.S.B conceived of the need and initial designs for the measurement campaign; J.A.T. performed much of the final analysis and modelling; T.P.R. performed the predictions of continental-scale ClNO2 production; and J.A.T. wrote the paper with significant input from all co-authors, especially S.S.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Discussion, Supplementary Notes and References, Supplementary Figures 1-10 with Legends, and Supplementary Table 1. (PDF 1691 kb)
Rights and permissions
About this article
Cite this article
Thornton, J., Kercher, J., Riedel, T. et al. A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature 464, 271–274 (2010). https://doi.org/10.1038/nature08905
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08905
This article is cited by
-
Increased night-time oxidation over China despite widespread decrease across the globe
Nature Geoscience (2023)
-
Natural short-lived halogens exert an indirect cooling effect on climate
Nature (2023)
-
Reactive halogens increase the global methane lifetime and radiative forcing in the 21st century
Nature Communications (2022)
-
Photodissociation of particulate nitrate as a source of daytime tropospheric Cl2
Nature Communications (2022)
-
A diurnal story of Δ17O(\(\rm{NO}_{3}^{-}\)) in urban Nanjing and its implication for nitrate aerosol formation
npj Climate and Atmospheric Science (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.