Abstract
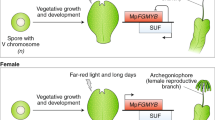
The flowering plant family Hydatellaceae was recently discovered to be allied to the ancient angiosperm lineage Nymphaeales (water lilies)1. Because of its critical phylogenetic position, members of the Hydatellaceae have the potential to provide insights into the origin and early diversification of angiosperms2. Here I report that Hydatella expresses several rare embryological features that, in combination, are found only in members of the Nymphaeales. At maturity, the female gametophyte is four-celled, four-nucleate and will produce a diploid endosperm, as is characteristic of most early divergent angiosperm lineages3,4. As with all members of the Nymphaeales, endosperm in Hydatella is minimally developed and perisperm is the major embryo-nourishing tissue within the seed5,6. Remarkably, Hydatella exhibits a maternal seed-provisioning strategy that is unique among flowering plants, but common to all gymnosperms7: pre-fertilization allocation of nutrients to the embryo-nourishing tissue. This exceptional case of pre-fertilization maternal provisioning of a seed in Hydatella may well be an apomorphic feature of Hydatellaceae alone but, given the newly discovered phylogenetic position of this family, potentially represents a plesiomorphic and transitional condition associated with the origin of flowering plants from gymnospermous ancestors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Saarela, J. M. et al. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature 446, 312–315 (2007)
Rudall, P. J. et al. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. Am. J. Bot. 94, 1073–1092 (2007)
Williams, J. H. & Friedman, W. E. Identification of diploid endosperm in an early angiosperm lineage. Nature 415, 522–525 (2002)
Tobe, H., Kimoto, Y. & Prakash, N. Development and structure of the female gametophyte in Austrobaileya scandens (Austrobaileyaceae). J. Plant Res. 120, 431–436 (2007)
Hamann, U. Neue Untersuchungen zur Embryologie und Systematik der Centrolepidaceae. Bot. Jahr. 96, 154–191 (1975)
Johri, B. M., Ambegaokar, K. B. & Srivastava, P. S. Comparative Embryology of Angiosperms. (McGraw-Hill, New York, 2002)
Friedman, W. E. & Carmichael, J. S. Heterochrony and developmental innovation: evolution of female gametophyte ontogeny in Gnetum, a highly apomorphic seed plant. Evolution 52, 1016–1030 (1998)
Mathews, S. & Donoghue, M. J. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286, 947–950 (1999)
Parkinson, C. L., Adams, K. L. & Palmer, J. D. Multigene analyses identify the three earliest lineages of extant flowering plants. Curr. Biol. 9, 1485–1488 (1999)
Qiu, Y.-L. et al. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402, 404–407 (1999)
Soltis, P. S., Soltis, D. E. & Chase, M. W. Angiosperm phylogeny inferred from multiple genes as a research tool for comparative biology. Nature 402, 402–404 (1999)
Graham, S. W. & Olmstead, R. G. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. Am. J. Bot. 87, 1712–1730 (2000)
Friedman, W. E. Embryological evidence for developmental lability during early angiosperm evolution. Nature 441, 337–340 (2006)
Hamann, U. Hydatellaceae – a new family of Monocotyledoneae. N.Z. J. Bot. 14, 193–196 (1976)
Hamann, U. in Families and Genera of Vascular Plants vol. IV (ed. Kubitzki, K.) 231–235 (Springer, Berlin, 1998)
Friedman, W. E., Gallup, W. N. & Williams, J. H. Female gametophyte development in Kadsura: implications for Schisandraceae, Austrobaileyales, and the early evolution of flowering plants. Int. J. Plant Sci. 164 (suppl.). S294–S305 (2003)
Friedman, W. E., Madrid, E. N. & Williams, J. H. Origin of the fittest and survival of the fittest: relating female gametophyte development to endosperm genetics. Int. J. Plant Sci. 169, 79–92 (2008)
Rudall, P. J. The nucellus and chalaza in monocotyledons: structure and systematics. Bot. Rev. 63, 140–184 (1997)
Schneider, E. L. Morphological studies of the Nymphaeaceae. IX. The seed of Barclaya longifolia Wall. Bot. Gaz. 139, 223–230 (1978)
Floyd, S. K. & Friedman, W. E. Developmental evolution of endosperm in basal angiosperms: evidence from Amborella (Amborellaceae), Nuphar (Nymphaeaceae), and Illicium (Illiciaceae). Plant Syst. Evol. 228, 153–169 (2001)
Stebbins, G. L. Flowering Plants: Evolution above the Species Level (Harvard Univ. Press, Cambridge, Massachusetts, 1974)
Tiffney, B. H. in Paleobotany, Paleoecology, and Evolution (ed. Niklas, K. J.) 193–230 (Praeger, New York, 1981)
Cocucci, A. E. Estudios en el género Prosopanche (Hydnoraceae). III Embriologia. Kurtziana 9, 19–39 (1976)
Rudall, P. J. & Furness, C. A. Systematics of Acorus: ovule and anther. Int. J. Plant Sci. 158, 640–651 (1997)
Floyd, S. K. & Friedman, W. E. Evolution of endosperm developmental patterns among basal flowering plants. Int. J. Plant Sci. 161 (suppl.). S57–S81 (2000)
Prakash, N. & Bak, H. K. Flower and fruit development in Piper nigrum L. cv. Kuching. Malays. J. Sci. 7, 11–19 (1982)
Prakash, N. in Plant Form and Function (eds Bhatia, B., Shukla, A. K. & Sharma, H. L.) 207–216 (Angkor, New Delhi, 1998)
Johnson, D. S. On the development of Saururus cernuus L. Bull. Torrey Bot. Club 27, 365–372 (1900)
Shamrov, I. I. in Embryology of Flowering Plants (ed. Batygina, T. B.) 169–170 (Science Publishers, Enfield, New Hampshire, 2006)
Doyle, J. A. in Early Evolution of Flowers (eds Endress, P. K. & Friis, E. M.) 7–29 (Springer, Vienna, 1994)
Acknowledgements
I thank: P. Champion and A. Drinnan for collecting plant materials; S. Holloway for histological work; S. Renner for translation of the embryological studies of U. Hamaan; and P. Diggle, L. Hufford, J. Williams and R. Robichaux for feedback on this manuscript. This work was supported by a National Science Foundation Research Grant.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure
The file contains Supplementary Figure with Legend showing longitudinal section of recently fertilized ovule of Hydatella inconspicua. A pollen tube that entered the ovule through the micropyle formed by the two integuments can be clearly seen. The zygote contains a prominent vacuole and the primary endosperm nucleus is situated at the base of the former female gametophyte. Upper grey box contains digital superposition of pollen tube from adjacent histological section. Lower grey box contains digital superposition of primary endosperm from adjacent histological section. Bar = 10 μm. pen = primary endosperm nucleus; ps = perisperm; pt = pollen tube; z = zygote. (PDF 2617 kb)
Rights and permissions
About this article
Cite this article
Friedman, W. Hydatellaceae are water lilies with gymnospermous tendencies. Nature 453, 94–97 (2008). https://doi.org/10.1038/nature06733
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06733
This article is cited by
-
The evolution of imprinting in plants: beyond the seed
Plant Reproduction (2021)
-
Why the monophyly of Nymphaeaceae currently remains indeterminate: an assessment based on gene-wise plastid phylogenomics
Plant Systematics and Evolution (2019)
-
Transcriptomics of manually isolated Amborella trichopoda egg apparatus cells
Plant Reproduction (2019)
-
Water lilies as emerging models for Darwin’s abominable mystery
Horticulture Research (2017)
-
Multidisciplinary studies of the diversity and evolution in river-weeds
Journal of Plant Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.