Abstract
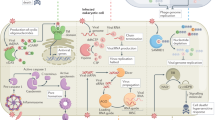
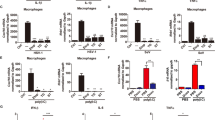
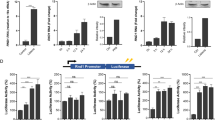
Vertebrate innate immunity provides a first line of defence against pathogens such as viruses and bacteria. Viral infection activates a potent innate immune response, which can be triggered by double-stranded (ds)RNA produced during viral replication1,2,3. Here, we report that mammalian cells lacking the death-domain-containing protein FADD4,5 are defective in intracellular dsRNA-activated gene expression, including production of type I (α/β) interferons, and are thus very susceptible to viral infection. The signalling pathway incorporating FADD is largely independent of Toll-like receptor 3 and the dsRNA-dependent kinase PKR, but seems to require receptor interacting protein 1 as well as Tank-binding kinase 1-mediated activation of the transcription factor IRF-3. The requirement for FADD in mammalian host defence is evocative of innate immune signalling in Drosophila, in which a FADD-dependent pathway responds to bacterial infection by activating the transcription of antimicrobial genes6. These data therefore suggest the existence of a conserved pathogen recognition pathway in mammalian cells that is essential for the optimal induction of type I interferons and other genes important for host defence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003)
Medzhitov, R. & Janeway, C. Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 8, 452–456 (2000)
Taniguchi, T. & Takaoka, A. The interferon-α/β system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14, 111–116 (2002)
Chinnaiyan, A. M., O'Rourke, K., Tewari, M. & Dixit, V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 (1995)
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998)
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003)
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998)
Balachandran, S. et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J. Virol. 74, 1513–1523 (2000)
Varfolomeev, E. E. et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998)
Muzio, M. et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85, 817–827 (1996)
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002)
Williams, B. R. PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999)
Balachandran, S. et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–141 (2000)
Chu, W. M. et al. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11, 721–731 (1999)
Diebold, S. S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324–328 (2003)
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001)
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nature Immunol. 4, 161–167 (2003)
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003)
Hemmi, H. et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004)
Honda, K. et al. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl Acad. Sci. USA 100, 10872–10877 (2003)
Hoebe, K. et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nature Immunol. 4, 1223–1229 (2003)
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004)
Leulier, F., Vidal, S., Saigo, K., Ueda, R. & Lemaitre, B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr. Biol. 12, 996–1000 (2002)
Naitza, S. et al. The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity 17, 575–581 (2002)
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998)
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nature Immunol. 5, 503–507 (2004)
Wathelet, M. G. et al. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1, 507–518 (1998)
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003)
Fitzgerald, K. A. et al. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunol. 4, 491–496 (2003)
McWhirter, S. M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl Acad. Sci. USA 101, 233–238 (2004)
Acknowledgements
We are grateful to W.-C. Yeh, N. Kelliher, D. Wallach, J. Inoue, J. Durbin, J. Bell, K. Mossman, E. Harhaj, M. Karin, B. Williams and S. Akira for fibroblasts, and T. Maniatis, J. Hiscott and N. Reich and for plasmid constructs. We also thank G. Spruill, M. Fallahi and T. Andrew for technical assistance. This work was supported by DARPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Absence of FADD sensitizes cells to infection by Encephalomyocarditis Virus (EMCV) and Influenza Virus (FLU). (JPG 82 kb)
Supplementary Figure 2
Caspase 8 is not required for protection against VSV. (JPG 96 kb)
Supplementary Figure 3
Normal IFN signalling in the absence of FADD. (JPG 23 kb)
Supplementary Figure 4
Confirmation of a requirement for FADD for optimal induction of IFN-β following intracellular dsRNA treatment. (JPG 37 kb)
Supplementary Figure 5
TLR3 and PKR independent signalling by intracellular dsRNA. (JPG 72 kb)
Supplementary Figure 6
Analysis of RIPK1 -/- MEFs. (JPG 37 kb)
Supplementary Figure 7
Quantitation of cell death and virus yield from Tbk1/Ikkδ+/+ and Tbk1/Ikkδ-/-, as well as Irf3+/+ and Irf3-/- MEFs. (JPG 51 kb)
Supplementary Figure 8
FADD, RIP1 and IRF-3 are required for IFN-α production in 293 cells. (JPG 79 kb)
Supplementary Figure 9
The RIP/FADD/TBK-1 (RIFT) cascade. (JPG 29 kb)
Supplementary Figure Legends
Legends to accompany the above figures. (DOC 55 kb)
Rights and permissions
About this article
Cite this article
Balachandran, S., Thomas, E. & Barber, G. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432, 401–405 (2004). https://doi.org/10.1038/nature03124
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03124
This article is cited by
-
Differences in IFNβ secretion upon Rab1 inactivation in cells exposed to distinct innate immune stimuli
Cellular & Molecular Immunology (2021)
-
Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study
Signal Transduction and Targeted Therapy (2020)
-
Therapeutic targeting of the PI4K2A/PKR lysosome network is critical for misfolded protein clearance and survival in cancer cells
Oncogene (2020)
-
Novel insights into the host immune response of chicken Harderian gland tissue during Newcastle disease virus infection and heat treatment
BMC Veterinary Research (2018)
-
OTULIN limits cell death and inflammation by deubiquitinating LUBAC
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.