Abstract
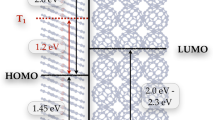
The ultrafast timescale of electron transfer processes is crucial to their role in many biological systems and technological devices. In dye-sensitized solar cells1,2,3,4, the electron transfer from photo-excited dye molecules to nanostructured semiconductor substrates needs to be sufficiently fast to compete effectively against loss processes and thus achieve high solar energy conversion efficiencies4. Time-resolved laser techniques indicate an upper limit of 20 to 100 femtoseconds5,6,7,8,9 for the time needed to inject an electron from a dye into a semiconductor, which corresponds to the timescale on which competing processes such as charge redistribution10,11 and intramolecular thermalization of excited states12,13,14 occur. Here we use resonant photoemission spectroscopy, which has previously been used to monitor electron transfer in simple systems with an order-of-magnitude improvement in time resolution15,16, to show that electron transfer from an aromatic adsorbate to a TiO2 semiconductor surface can occur in less than 3 fs. These results directly confirm that electronic coupling of the aromatic molecule to its substrate is sufficiently strong to suppress competing processes17.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hagfeldt, A. & Grätzel, M. Molecular photovoltaics. Acc. Chem. Res. 33, 269–277 (2000)
Vlček, A. Jr The life and times of excited states of organometallic and coordination compounds. Coord. Chem. Rev. 200–202, 933–977 (2000)
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001)
Miller, R. J. D., McLendon, G. L., Nozik, A. J., Schmickler, W. & Willig, F. Surface Electron Transfer Processes (VCH Publishers, New York, 1995)
Tachibana, Y., Moser, J. E., Grätzel, M., Klug, D. R. & Durrant, J. R. Subpicosecond interfacial charge separation in dye-sensitized nanocrystalline titanium dioxide films. J. Phys. Chem. 100, 20056–20062 (1996)
Hannappel, T., Burfeindt, B., Storck, W. & Willig, F. Measurement of ultrafast photoinduced electron transfer from chemically anchored Ru-dye molecules into empty electronic states in a colloidal anatase TiO2 film. J. Phys. Chem. B 101, 6799–6802 (1997)
Asbury, J. B., Hao, E., Wang, Y., Ghosh, H. N. & Lian, T. Ultrafast electron transfer dynamics from molecular adsorbates to semiconductor nanocrystalline thin films. J. Phys. Chem. B 105, 4545–4557 (2001)
Heimer, T. A., Heilweil, E. J., Bignozzi, C. A. & Meyer, G. J. Electron injection, recombination, and halide oxidation dynamics at dye-sensitized metal oxide interfaces. J. Phys. Chem. A 104, 4256–4262 (2000)
Benkö, G., Kallioinen, J., Korppi-Tommola, J. E. I., Yartsev, A. P. & Sundström, V. Photoinduced ultrafast dye-to semiconductor electron injection from nonthermalized and thermalized donor states. J. Am. Chem. Soc. 124, 489–493 (2002)
Yeh, A. T., Shank, C. V. & McCusker, J. K. Ultrafast electron localization dynamics following photo-induced charge transfer. Science 289, 935–938 (2000)
Tachibana, Y., Haque, S. A., Mercer, I. P., Durrant, J. R. & Klug, D. R. Electron injection and recombination in dye sensitized nanocrystalline titanium dioxide films: A comparison of ruthenium bipyridyl and porphyrin sensitizer dyes. J. Phys. Chem. B 104, 1198–1205 (2000)
Damrauer, N. H. et al. Femtosecond dynamics of excited-state evolution in [Ru(bpy)3]2+. Science 275, 54–57 (1997)
Blanchet, V., Zgierski, M. Z., Seideman, T. & Stolow, A. Discerning vibronic molecular dynamics using time-resolved photoelectron spectroscopy. Nature 401, 52–54 (1999)
Willig, F., Zimmermann, C., Ramakrishna, S. & Storck, W. Ultrafast dynamics of light-induced electron injection from a molecular donor into the wide conduction band of a semiconductor as acceptor. Electrochim. Acta 45, 4565–4575 (2000)
Wurth, W. & Menzel, D. Ultrafast electron dynamics at surfaces probed by resonant Auger spectroscopy. Chem. Phys. 251, 141–149 (2000)
Brühwiler, P. A., Karis, O. & Mårtensson, N. Charge transfer dynamics studied using resonant core spectroscopies. Rev. Mod. Phys. 74, 703–740 (2002)
Lanzafame, J. M., Palese, S., Wang, D., Miller, R. J. D. & Muenter, A. A. Ultrafast nonlinear optical studies of surface reaction dynamics: Mapping the electron trajectory. J. Phys. Chem. 98, 11020–11033 (1994)
Nazeeruddin, M. K. et al. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 115, 6382–6390 (1993)
Rensmo, H. et al. XPS studies of Ru-polypyridine complexes for solar cell applications. J. Chem. Phys. 111, 2744–2750 (1999)
Patthey, L. et al. Adsorption of bi-isonicotinic acid on rutile TiO2(110). J. Chem. Phys. 110, 5913–5918 (1999)
Persson, P. & Lunell, S. Binding of bi-isonicotinic acid to anatase TiO2(101). Solar Energy Mater. Solar Cells 63, 139–148 (2000)
Cronemeyer, D. C. Electrical and optical properties of rutile single crystals. Phys. Rev. 87, 876–886 (1952)
O'Regan, B. & Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991)
Persson, P. et al. N 1s x-ray absorption study of the bonding interaction of bi-isonicotinic acid adsorbed on rutile TiO2(110). J. Chem. Phys. 112, 3945–3948 (2000)
Chang, E. K., Rohlfing, M. & Louie, S. G. Excitons and optical properties of alpha-quartz. Phys. Rev. Lett. 85, 2613–2616 (2000)
Sandell, A. et al. Bonding of an isolated K atom to a surface: Experiment and theory. Phys. Rev. Lett. 78, 4994–4997 (1997)
Lu, H., Prieskorn, J. N. & Hupp, J. T. Fast interfacial electron transfer: Evidence for inverted region kinetic behavior. J. Am. Chem. Soc. 115, 4927–4928 (1993)
Ramakrishna, S. & Willig, F. Pump-probe spectroscopy of ultrafast electron injection from the excited state of an anchored chromophere to a semiconductor surface in UHV: A theoretical model. J. Phys. Chem. B 104, 68–77 (2000)
Petersson, Å., Ratner, M. & Karlsson, H. O. Injection time in the metal oxide-molecule interface calculated within the tight-binding model. J. Phys. Chem. B 104, 8498–8502 (2000)
Acknowledgements
We are grateful for financial support to the Consortium on Clusters and Ultrafine Particles and to the ATOMICS Consortium, which are funded by Stiftelsen for Strategisk Forskning, to Göran Gustafssons Stiftelse, and to Vetenskapsrådet. We acknowledge the Swedish National Supercomputer Centre (NSC) for computer time, and the Group for Numerically-intensive Computations of the IBM Research Laboratory Zürich and the Abteilung Parrinello of MPI Stuttgart for help with the calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Schnadt, J., Brühwiler, P., Patthey, L. et al. Experimental evidence for sub-3-fs charge transfer from an aromatic adsorbate to a semiconductor. Nature 418, 620–623 (2002). https://doi.org/10.1038/nature00952
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00952
This article is cited by
-
Attosecond science based on high harmonic generation from gases and solids
Nature Communications (2020)
-
Gold catalysts containing interstitial carbon atoms boost hydrogenation activity
Nature Communications (2020)
-
Encroachment of shrubs into subalpine grasslands in the Pyrenees changes the plant-soil stoichiometry spectrum
Plant and Soil (2020)
-
Directional sub-femtosecond charge transfer dynamics and the dimensionality of 1T-TaS2
Scientific Reports (2019)
-
An ultrasensitive photoelectrochemical platform for quantifying photoinduced electron-transfer properties of a single entity
Nature Protocols (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.