Abstract
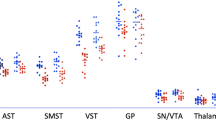
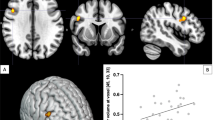
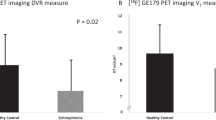
A common polymorphism (val158met) in the gene encoding catechol-O-methyltransferase (COMT) has been shown to affect dopamine (DA) tone in cortex and cortical functioning. D1 receptors are the main DA receptors in the cortex, and studies have shown that decreased levels of cortical DA are associated with upregulation of D1 receptor availability, as measured with the positron-emission tomography (PET) radiotracer [11C]NNC112. We compared [11C]NNC 112 binding in healthy volunteers homozygous for the Val allele compared with Met carriers. Subjects were otherwise matched for parameters known to affect [11C]NNC 112 binding. Subjects with Val/Val alleles had significantly higher cortical [11C]NNC 112 binding compared with Met carriers, but did not differ in striatal binding. These results confirm the prominent role of COMT in regulating DA transmission in cortex but not striatum, and the reliability of [11C]NNC 112 as a marker for low DA tone as previously suggested by studies in patients with schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
De Keyser J, Ebinger G, Vauquelin G . Evidence for a widespread dopaminergic innervation of the human cerebral neocortex. Neurosci Lett 1989; 104: 281–285.
Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L . Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994; 11: 245–256.
Lidow MD, Goldman-Rakic PS, Rakic P, Gallagher DW . Autoradiographic comparison of D1-specific binding of [3H]SCH39166 and [3H]SCH23390 in the primate cerebral cortex. Brain Res 1990; 537: 349–354.
Dawson TM, McCabe RT, Stensaas SS, Wamsley JK . Autoradiographic evidence of [3H]SCH23390 binding sites in human prefrontal cortex (Brodmann's area 9). J Neurochem 1987; 49: 789–796.
Lawtho D, Hirsch JC, Crepel F . Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res 1994; 21: 151–160.
Seamans JK, Gorelova N, Durstewitz D, Yang CR . Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 2001; 21: 3628–3638.
Yang CR, Seamans JK, Gorelova N . Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology 1999; 21: 161–194.
Yang CR, Seamans JK . Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic–somatic signal integration. J Neurosci 1996; 16: 1922–1935.
Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22: 3708–3719.
Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A . Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology 2003; 28: 1703–1711.
Jentsch JD, Taylor JR, Elsworth JD, Redmond Jr DE, Roth RH . Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience 1999; 90: 823–832.
Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino EF . Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in prefrontal cortex of conscious monkeys. Neuropsychopharmacology 2005; 30: 1861–1869.
Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M et al. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 2005; 162: 2352–2359.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI . Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 1998; 18: 2697–2708.
Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A . Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 2001; 432: 119–136.
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM . Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996; 6: 243–250.
Chen JS, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75: 807–821.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98: 6917–6922.
Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS et al. Executive subprocesses in working memory—relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 2003; 60: 889–896.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95: 9991–9996.
Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J et al. Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. J Nucl Med 1998; 39: 2061–2068.
Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M et al. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 2000; 20: 225–243.
Woods RP, Mazziotta JC, Cherry SR . MRI–PET registration with automated algorithm. J Comput Assist Tomogr 1993; 17: 536–546.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D-2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057.
Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22: 3708–3719.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Giedde A, Gunn RN et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539.
Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L . Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994; 11: 245–256.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95: 9991–9996.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ . Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 2004; 24: 5331–5335.
Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci 2005; 25: 5038–5045.
Bertolino A, Caforio G, Blasi G, Rampino A, Sinibaldi L, Douzgou S et al. COMT Val158Met polymorphism predicts negative symptoms response to treatment with olanzapine in schizophrenia. Biol Psychiatry 2007; 61: 13S.
Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry 2006; 63: 1396–1406.
Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 2003; 116: 127–137.
Masuda M, Tsunoda M, Imai K . High-performance liquid chromatography-fluorescent assay of catechol-O-methyltransferase activity in rat brain. Anal Bioanal Chem 2003; 376: 1069–1073.
Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu XY, Laruelle M et al. [C-11] NNC 112 selectivity for dopamine D-1 and serotonin 5-HT2A receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab 2007; 27: 1733–1741.
Li T, Sham PC, Vallada H, Xie T, Tang X, Murray RM et al. Preferential transmission of the high activity allele of COMT in schizophrenia. Psychiatr Genet 1996; 6: 131–133.
Herken H, Erdal ME . Catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association between symptomatology and prognosis. Psychiatr Genet 2001; 11: 105–109.
Ohmori O, Shinkai T, Kojima H, Terao T, Suzuki T, Mita T et al. Association study of a functional catechol-O-methyltransferase gene polymorphism in Japanese schizophrenics. Neurosci Lett 1998; 243: 109–112.
Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry 1996; 153: 268–270.
de Chaldee M, Corbex M, Campion D, Jay M, Samolyk D, Petit M et al. No evidence for linkage between COMT and schizophrenia in a French population. Psychiatry Res 2001; 102: 87–90.
Wei J, Hemmings GP . Lack of evidence for association between the COMT locus and schizophrenia. Psychiatr Genet 1999; 9: 183–186.
Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry 2005; 57: 139–144.
Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry 2006; 11: 867–877.
Acknowledgements
We acknowledge the excellent technical support provided by Elizabeth Hackett in PET acquisition, John Castrillon in radioligand preparation, Jennifer Bae in plasma metabolite analysis and Erica Scher in data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slifstein, M., Kolachana, B., Simpson, E. et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry 13, 821–827 (2008). https://doi.org/10.1038/mp.2008.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2008.19
Keywords
This article is cited by
-
Cortical thickness mediates the relationship between DRD2 C957T polymorphism and executive function across the adult lifespan
Brain Structure and Function (2021)
-
Analysis of COMT Val158Met polymorphisms and methylation in Chinese male schizophrenia patients with homicidal behavior
International Journal of Legal Medicine (2018)
-
Predicting the ergogenic response to methylphenidate
European Journal of Applied Physiology (2018)
-
Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex
Neuropsychopharmacology (2017)
-
COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review
The Pharmacogenomics Journal (2016)