Abstract
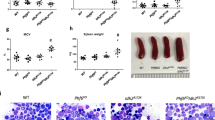
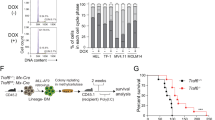
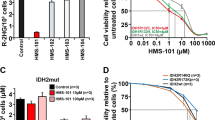
Canonical mutations in IDH1 and IDH2 produce high levels of the R-enantiomer of 2-hydroxyglutarate (R-2HG), which is a competitive inhibitor of α-ketoglutarate (αKG)-dependent enzymes and a putative oncometabolite. Mutant IDH1 collaborates with HoxA9 to induce monocytic leukemia in vivo. We used two mouse models and a patient-derived acute myeloid leukemia xenotransplantation (PDX) model to evaluate the in vivo transforming potential of R-2HG, S-2HG and αKG independent of the mutant IDH1 protein. We show that R-2HG, but not S-2HG or αKG, is an oncometabolite in vivo that does not require the mutant IDH1 protein to induce hyperleukocytosis and to accelerate the onset of murine and human leukemia. Thus, circulating R-2HG acts in a paracrine manner and can drive the expansion of many different leukemic and preleukemic clones that may express wild-type IDH1, and therefore can be a driver of clonal evolution and diversity. In addition, we show that the mutant IDH1 protein is a stronger oncogene than R-2HG alone when comparable intracellular R-2HG levels are achieved. We therefore propose R-2HG-independent oncogenic functions of mutant IDH1 that may need to be targeted in addition to R-2HG production to exploit the full therapeutic potential of IDH1 inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009; 361: 1058–1066.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360: 765–773.
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 2011; 224: 334–343.
Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012; 17: 72–79.
Thol F, Damm F, Wagner K, Gohring G, Schlegelberger B, Hoelzer D et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood 2010; 116: 614–616.
Wagner K, Damm F, Gohring G, Gorlich K, Heuser M, Schafer I et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol 2010; 28: 2356–2364.
Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol 2010; 28: 3636–3643.
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010; 17: 225–234.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010; 465: 966.
Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 2010; 207: 339–344.
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 2009; 324: 261–265.
Rose NR, McDonough MA, King ON, Kawamura A, Schofield CJ . Inhibition of 2-oxoglutarate dependent oxygenases. Chem Soc Rev 2011; 40: 4364–4397.
Loenarz C, Schofield CJ . Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol 2008; 4: 152–156.
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011; 19: 17–30.
Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 2011; 12: 463–469.
Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S et al. Transformation by the (R-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 2012; 483: 484–488.
Heuser M, Araujo Cruz MM, Goparaju R, Chaturvedi A . Enigmas of IDH mutations in hematology/oncology. Exp Hematol 2015; 43: 685–697.
Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 2012; 488: 656–659.
Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Yun H, Gorlich K et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood 2013; 122: 2877–2887.
Heuser M, Yun H, Berg T, Yung E, Argiropolous B, Kuchenbauer F et al. Cell of origin in AML: Susceptibility to MN1-induced transformation is regulated by the MEIS1/AbdB-like HOX protein complex. Cancer Cell 2011; 20: 39–52.
Heuser M, Sly LM, Argiropoulos B, Kuchenbauer F, Lai C, Weng A et al. Modeling the functional heterogeneity of leukemia stem cells: role of STAT5 in leukemia stem cell self-renewal. Blood 2009; 114: 3983–3993.
Struys EA, Jansen EE, Verhoeven NM, Jakobs C . Measurement of urinary d- and l-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-l-tartaric anhydride. Clin Chem 2004; 50: 1391–1395.
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Kauffmann A, Gentleman R, Huber W . arrayQualityMetrics—a bioconductor package for quality assessment of microarray data. Bioinformatics 2009; 25: 415–416.
Gautier L, Cope L, Bolstad BM, Irizarry RA . affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004; 20: 307–315.
Smyth GK . LIMMA: Linear Models for Microarray Data Bioinformatics and Computational Biology Solutions Using R and Bioconductor, Part V. Springer: New York, NY, USA,, 2005, Mpp 397–420.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Heuser M, Beutel G, Krauter J, Dohner K, von Neuhoff N, Schlegelberger B et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood 2006; 108: 3898–3905.
Heuser M, Yap DB, Leung M, de Algara TR, Tafech A, McKinney S et al. Loss of MLL5 results in pleiotropic hematopoietic defects, reduced neutrophil immune function, and extreme sensitivity to DNA demethylation. Blood 2009; 113: 1432–1443.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 2001; 25: 402–408.
Smith ZD, Gu H, Bock C, Gnirke A, Meissner A . High-throughput bisulfite sequencing in mammalian genomes. Methods 2009; 48: 226–232.
Xi Y, Li W . BSMAP: whole genome bisulfite sequence MAPping program. BMC Bioinform 2009; 10: 232.
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009; 462: 315–322.
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012; 483: 474–478.
Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C et al. (R-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013; 339: 1621–1625.
Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med 2015; 21: 178–184.
Kats LM, Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell 2014; 14: 329–341.
Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS, Jakobs C . Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 2012; 35: 571–587.
Acknowledgements
We acknowledge assistance of the Cell Sorting Core Facility of Hannover Medical School supported, in part, by the Braukmann-Wittenberg-Herz-Stiftung and the Deutsche Forschungsgemeinschaft. We thank the staff of the Central Animal Facility of Hannover Medical School, Vishwas Sharma, Razif Gabdoulline, Michael Morgan, Silke Glowotz, Nicole Ernst and Martin Wichmann for their support on this project. We would also like to thank Dr Florian Kuchenbauer for providing the MLL-AF9 construct. This study was supported by an ERC grant under the European Union’s Horizon 2020 research and innovation program (No. 638035), by Grants 110284, 110287, 110292 and 111267 from Deutsche Krebshilfe; Grant DJCLS R13/14 from the Deutsche José Carreras Leukämie-Stiftung eV; the German Federal Ministry of Education and Research Grant 01EO0802 (IFB-Tx); DFG Grant HE 5240/5-1 and HE 5240/6-1; grants from Dieter-Schlag Stiftung, and a HiLF grant from Hannover Medical School awarded (to AC).
Author contributions
AC and MH designed the research; AC, MMAC, NJ, ASh, RGo, ASc, KG, RS, ES, EJ, CR, CM, RGe and GG performed the research; FT, AG and MH contributed patient samples and clinical data; AC, MMAC, ASc, CR, RGe and MH analyzed the data. AC and MH wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Chaturvedi, A., Araujo Cruz, M., Jyotsana, N. et al. Enantiomer-specific and paracrine leukemogenicity of mutant IDH metabolite 2-hydroxyglutarate. Leukemia 30, 1708–1715 (2016). https://doi.org/10.1038/leu.2016.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.71
This article is cited by
-
Clonal evolution in leukemia: preleukemia, evolutionary models, and clinical implications
Genome Instability & Disease (2023)
-
The return of quiescence metabolites
Nature Cell Biology (2021)
-
IDH1/2 mutations in acute myeloid leukemia patients and risk of coronary artery disease and cardiac dysfunction—a retrospective propensity score analysis
Leukemia (2021)
-
Integrated genomic-metabolic classification of acute myeloid leukemia defines a subgroup with NPM1 and cohesin/DNA damage mutations
Leukemia (2021)
-
In vivo efficacy of mutant IDH1 inhibitor HMS-101 and structural resolution of distinct binding site
Leukemia (2020)