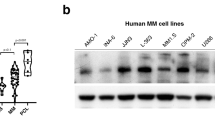
Abstract
DLC1 (deleted in liver cancer 1), a tumor suppressor gene that encodes a RhoGTPase-activating protein, is recurrently downregulated or silenced in various solid tumors and hematological malignancies because of epigenetic modifications or genomic deletion. Here, we identified DLC1 promoter hypermethylation in 43 out of 44 multiple myeloma (MM) cell lines, which resulted in downregulation or silencing of DLC1 in 41 samples. High frequency of tumor-specific methylation and attenuation or silencing of DLC1 expression could serve as an independent diagnostic marker for MM. Combined treatment with demethylating and acetylating agents significantly elevated the expression of DLC1 and suppressed MM cell proliferation. Two cell lines exhibiting complete promoter methylation and the absence of DLC1 expression were transduced by an adenoviral vector containing DLC1 cDNA. In both cell lines, the reexpression of DLC1 inhibited myeloma cell invasion and migration, reduced RhoA activity and resulted in the reorganization of actin cytoskeleton. These results provide the first evidence for the antiproliferative effect of DLC1 in a hematological cancer and implicate RhoA pathway in suppression of MM migration and invasion. Given the myeloma cells sensitivity to the reactivation of DLC1 function, the potential for molecular targeted therapy of DLC1-mediated pathways as well as epigenetic therapies hold prospects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ et al. (eds). SEER Cancer Statistics Review, 1975–2005, National Cancer Institute: Bethesda, MD, 2007, http://seer.cancer.gov/csr 1975_2005/, based on November 2007 SEER data submission, posted to the SEER website, 2008.
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC . Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 2007; 7: 585–598.
Kyle RA, Rajkumar SV . Multiple myeloma. Blood 2008; 111: 2962–2972.
Bergsagel PL, Kuehl WM . Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol 2005; 23: 6333–6338.
Spink CF, Gray LC, Davies FE, Morgan GJ, Bidwell JL . Haplotypic structure across the I kappa B alpha gene (NFKBIA) and association with multiple myeloma. Cancer Lett 2007; 246: 92–99.
Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell 2006; 9: 313–325.
Kuehl WM, Bergsagel PL . Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer 2002; 2: 175–187.
Yuan BZ, Miller MJ, Keck CL, Zimonjic D, Thorgeirsson SS . Cloning, characterization and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res 1998; 58: 2196–2199.
Durkin ME, Yuan BZ, Zhou X, Zimonjic DB, Lowy DR, Thorgeirsson SS et al. DLC-1: a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med 2007; 11: 1185–1207.
Xue W, Krasnitz A, Lucito R, Sordella R, VanAelst L, Cordon-Cardo C et al. DLC1 is a chromosome 8p tumor suppressor whose loss promotes hepatocellular carcinoma. Genes Dev 2008; 22: 1439–1444.
Shi H, Guo J, Duff DJ, Rahmatpanah F, Chitima-Matsiga R, Al-Kuhlani M et al. Discovery of novel epigenetic markers in non-Hodgkin's lymphoma. Carcinogenesis 2007; 28: 60–70.
Taylor KH, Pena-Hernandez KE, Davis JW, Arthur GL, Duff DJ, Shi H et al. Large-scale CpG methylation analysis identifies novel candidate genes and reveals methylation hotspots in acute lymphoblastic leukemia. Cancer Res 2007; 67: 2617–2625.
Pike BL, Greiner TC, Wang X, Weisenburger DD, Hsu YH, Renaud G et al. DNA methylation profiles in diffuse large B-cell lymphoma and their relationship to gene expression status. Leukemia 2008; 22: 1035–1043.
Ying J, Li H, Murray P, Gao Z, Chen YW, Wang Y et al. Tumor-specific methylation of the 8p22 tumor suppressor gene DLC1 is an epigenetic biomarker for Hodgkin, nasal NK/T-cell and other types of lymphomas. Epigenetics 2007; 2: 15–21.
Song YF, Xu R, Zhang XH, Chen BB, Chen Q, Chen YM et al. High-frequency promoter hypermethylation of the deleted in liver cancer-1 gene in multiple myeloma. J Clin Pathol 2006; 59: 947–951.
Xu L, Hatjiharissi E, Ciccareli BT, Roccaro AM, Adamia S, Sacco A, Hunter ZR et al. Expression of the deleted in liver cancer-1 gene is regulated by DNA methylation and is a target for therapy in Waldenstrom's macroglobulinemia. J Clin Oncol 2008; 26: 15S (Supplement), 19505.
Strutt DI, Weber U, Mlodzik M . The role of RhoA in tissue polarity and Frizzled signalling. Nature 1997; 387: 292–295.
Thompson MD, Monga SP . WNT/beta-catenin signaling in liver health and disease. Hepatology 2007; 45: 1298–1305.
Qiang YW, Walsh K, Yao L, Kedei N, Blumberg PM, Rubin JS et al. Wnts induce migration and invasion of myeloma plasma cells. Blood 2005; 106: 1786–1793.
Guan M, Tripathi V, Zhou X, Popescu NC . Adenovirus-mediated restoration of the expression of the tumor suppressor gene DLC1 inhibits the proliferation and tumorigenicity of aggressive, androgen-independent human prostate cancer cell lines: prospects for gene therapy. Cancer Gene Therapy 2008; 15: 371–378.
Zhou X, Zimonjic DB, Park S-W, Yang X-Y, Durkin ME, Popescu NC . DLC1 suppresses distant dissemination of human hepatocellular carcinoma cells in nude mice through reduction of RhoA GTPase activity, actin cytoskeletal disruption and down regulation of genes involved in metastasis. Int J Oncol 2008; 32: 1285–1291.
Guan M, Zhou X, Soulitzis N, Spandidos DA, Popescu NC . Aberrant methylation and deacetylation of deleted in liver cancer-1 gene in prostate cancer: potential clinical applications. Clin Cancer Res 2006; 12: 1412–1419.
Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE . Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179: 1634–1647.
Qiang YW, Yao L, Tosato G, Rudikoff S . Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood 2004; 103: 301–308.
Durkin ME, Avner MR, Huh CG, Yuan BZ, Thorgeirsson SS, Popescu NC . DLC-1, a Rho GTPase-activating protein with tumor suppressor function, is essential for embryonic development. FEBS Lett 2005; 579: 1191–1196.
Jones PA, Baylin SB . The fundamental role of epigenetic events in cancer. Nat Rev Genet 2003; 3: 415–428.
Herman JG, Baylin SB . Mechanisms of disease: gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003; 349: 2042–2054.
Galm O, Wilop S, Reichelt J, Jost E, Gehbauer G, Herman JG et al. DNA methylation changes in multiple myeloma. Leukemia 2004; 18: 1687–1692.
Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res 2006; 66: 6361–6369.
Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G et al. Preferential response of cancer cells to zebularine. Cancer Cell 2004; 6: 151–158.
Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid (SAHA)) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008; 111: 1060–1066.
Lahoz A, Hall A . DLC1: a significant GAP in the cancer genome. Genes Dev 2008; 22: 1724–1730.
Stafford LJ, Vaidya KS, Welch DR . Metastasis suppressor genes in cancer. Int J Biochem Cell Biol 2008; 40: 874–891.
Goodison S, Yuan J, Sloan D, Kim R, Li C, Popescu NC et al. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res 2005; 65: 6042–6053.
Santos E, Tronick SR, Aaronson SA, Pulciani S, Barbacid M . T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature 1982; 298: 343–347.
Jaffe AB, Hall A . Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21: 247–269.
Sahai EM, Marshall CJ . RHO-GTPases and cancer. Nat Rev Cancer 2002; 2: 133–142.
Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC . Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays 2005; 27: 602–613.
Wong CM, Lee JM, Ching YP, Jin DY, Ng IO . Genetic and epigenetic alterations of DLC-1 gene in hepatocellular carcinoma. Cancer Res 2003; 63: 7646–7651.
Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC, Braverman R et al. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci USA 2007; 104: 9012–9017.
Healy KD, Hodgson L, Kim TY, Shutes A, Maddileti S, Juliano RL et al. DLC-1 suppresses non-small cell lung cancer growth and invasion by RhoGAP-dependent and independent mechanisms. Mol Carcinog 2007; 47: 326–337.
Gabrea A, Martelli ML, Qi Y, Roschke A, Barlogie B, Shaughnessy JD et al. Secondary genomic rearrangements involving immunoglobulin or MYC loci show similar prevalences in hyperdiploid and nonhyperdiploid myeloma tumors. Genes Chromosomes Cancer 2008; 47: 573–590.
Saci A, Carpenter CL . RhoA GTPase regulates B cell receptor signaling. Mol Cell 2005; 17: 205–214.
Hall A . Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–514.
Wheeler AP, Ridley AJ . Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 2004; 301: 43–49.
Menu E, Braet F, Timmers M, Van Rieta I, Van Camp B, Vanderkerken K . The F-actin content of multiple myeloma cells as a measure of their migration. Ann N Y Acad Sci 2002; 973: 124–134.
Qiang YW, Endo Y, Rubin JS, Rudikoff S . Wnt signaling in B-cell neoplasia. Oncogene 2003; 22: 1536–1545.
Yuan BZ, Jefferson AM, Millecchia L, Popescu NC, Reynolds SH . Morphological changes and nuclear translocation of DLC1 tumor suppressor protein precede apoptosis in human non-small cell lung carcinoma cells. Exp Cell Res 2007; 313: 3868–3880.
Wong CC, Wong CM, Ko FC, Chan LK, Ching YP, Yam JW et al. Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS ONE 2008; 3: e2779.
Acknowledgements
This research was supported by the Intramural Research Program of the National Cancer Institute, NIH. We thank Dr Michael Kuehl (Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA) for providing DNA and RNA samples isolated from multiple myeloma cell lines, critical reading of the manuscript and most helpful suggestions. We also thank to Dr Sheng Chen for kindly providing plasma cells samples from healthy donors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ullmannova-Benson, V., Guan, M., Zhou, X. et al. DLC1 tumor suppressor gene inhibits migration and invasion of multiple myeloma cells through RhoA GTPase pathway. Leukemia 23, 383–390 (2009). https://doi.org/10.1038/leu.2008.285
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.285
Keywords
This article is cited by
-
DLC1 deficiency at diagnosis predicts poor prognosis in acute myeloid leukemia
Experimental Hematology & Oncology (2022)
-
Proteasomal turnover of the RhoGAP tumor suppressor DLC1 is regulated by HECTD1 and USP7
Scientific Reports (2022)
-
Frequent Downregulation and Promoter Hypermethylation of DLC1: Relationship with Clinical Outcome in Gallbladder Cancer
Journal of Gastrointestinal Cancer (2022)
-
Impact of clinical features, cytogenetics, genetic mutations, and methylation dynamics of CDKN2B and DLC-1 promoters on treatment response to azacitidine
Annals of Hematology (2020)
-
The mechanistic role of epigenetic in multiple myeloma
Comparative Clinical Pathology (2016)