Abstract
Plasminogen activator inhibitor-1 (PAI-1) is an acute-phase protein known to be involved in alcoholic liver disease and hepatic fibrosis. In the present study, the hypothesis that PAI-1 is causally involved in the onset of fructose-induced hepatic steatosis was tested in a mouse model. Wild-type C57BL/6J and PAI-1−/− mice were fed with 30% fructose solution or water for 8 weeks. Markers of hepatic steatosis, expression of PAI-1, apolipoprotein B (ApoB), cluster of differentiation 1d (CD1d), markers of natural killer T (NKT) cells, protein levels of phospho-c-Met and tumor necrosis factor-α (TNF-α) were determined. Activity of the microsomal triglyceride transfer protein (MTTP) was measured in liver tissue. In comparison with water controls, chronic intake of 30% fructose solution caused a significant increase in hepatic triglycerides, PAI-1 expression and plasma alanine aminotransferase levels in wild-type mice. This effect of fructose feeding was markedly attenuated in PAI-1−/− mice. Despite no differences in portal endotoxin levels and hepatic TNF-α protein levels between fructose-fed groups, the protective effect of the loss of PAI-1 against the onset of fructose-induced steatosis was associated with a significant increase in phospho-c-Met, phospho Akt, expression of ApoB and activity of MTTP in livers of PAI-1−/− mice in comparison with fructose-fed wild types. Moreover, in PAI-1−/− mice, expressions of CD1d and markers of CD1d-reactive NKT cells were markedly higher than in wild-type mice; however, expression of markers of activation of CD1d-reactive NKT cells (eg, interleukin-15 and interferon-γ) were only found to be increased in livers of fructose-fed PAI-1−/− mice. Taken together, these data suggest that PAI-1 has a causal role in mediating the early phase of fructose-induced liver damage in mice through signaling cascades downstream of Kupffer cells and TNF-α.
Similar content being viewed by others
Main
Non-alcoholic fatty liver disease (NAFLD), which is associated with obesity, type 2 diabetes and dyslipidemia, has become a worldwide health problem during the last decades.1, 2, 3, 4 NAFLD encompasses a broad spectrum of pathological conditions ranging from simple steatosis to steatohepatitis, fibrosis and finally to cirrhosis.5, 6, 7, 8, 9 Molecular mechanisms involved in the pathogenesis of NAFLD still remain unclear and therapies are limited. A better understanding of the molecular mechanisms contributing to the development of the early stages of NAFLD is desirable to develop prevention and also new therapeutic strategies.
Throughout the last three decades, dietary fructose intake has markedly increased in the United States and in Europe, paralleling the prevalence of obesity and type 2 diabetes.10, 11, 12 Results of recent studies further suggest that a diet rich in fructose may also be a risk factor for the development of NAFLD in humans.13, 14, 15, 16 The hypothesis that fructose intake might be causally involved on the onset and progression of NAFLD is also bolstered by the results of several animal studies. For instance, it has been shown that in rodents a diet rich in fructose can cause steatosis and oxidative stress in the liver, as well as insulin resistance.17, 18, 19, 20 Results of our own group have suggested that the onset of hepatic steatosis caused by chronic moderate fructose intake (eg, 30% fructose solution as only fluid source) is associated with an increased translocation of endotoxin from the intestine into the portal vein, formation of reactive oxygen species (ROS) in the liver and an induction of hepatic tumor necrosis factor-α (TNF-α) expression;19, 21 however, despite the fact that markers of insulin resistance such as plasma RBP-4 levels were found to be elevated in this model,21 blood glucose levels did not differ between fructose-fed mice and controls.19 An increased expression of TNF-α has repeatedly been reported to be associated with the development of NAFLD in different animal models.22, 23, 24 Indeed, in line with the findings of other groups, we recently showed that TNF-α receptor 1−/− (TNFR1−/−) mice were protected from the onset of fructose-induced steatosis and also insulin resistance.25 However, mechanisms involved in the deleterious effects of TNF-α on the liver have not yet been fully understood.
Plasminogen activator inhibitor-1 (PAI-1) is an acute-phase protein, which lately has also been linked to the onset and to the progression of liver diseases of various etiologies (eg, alcohol and endotoxin; for overview, see ref. 26). For instance, we and others reported that patients with NAFLD have elevated hepatic and plasma levels of PAI-1 in comparison with controls.13, 27 Furthermore, in animal studies, it was shown that PAI-1 expression in the liver and plasma is increased in genetically obese mice.28 Ma et al29 showed that PAI-1−/− mice are protected not only from weight gain but also from marked hepatic lipid accumulation in a mouse model of high-fat/high-carbohydrate feeding. It has further been shown in animal models of acute alcohol-induced liver steatosis and in in vitro experiments that PAI-1 is involved in mediating the pivotal effect of TNF-α on the liver.30 However, whether PAI-1 is also causally involved in the onset of fructose-induced hepatic steatosis in mice has not been clarified. Starting from this background, the aim of the present study was to assess in a mouse model whether PAI-1 is also a critical factor in the onset of fructose-induced hepatic steatosis and if so, what the underlying mechanisms involved are.
MATERIALS AND METHODS
Animals and Treatments
Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Procedures were approved by the local Institutional Animal Care and Use Committee. The 6-week-old C57BL/6J and PAI-1−/− mice (Serpine1tm1Mlg ID: 1857287, Jackson, Bar Harbor, USA) were fed with plain water or water containing 30% fructose ad libitum for a period of 8 weeks. Water intake was assessed weekly twice, whereas food intake and body weight gain were assessed once a week. Animals were anesthetized with 80 mg ketamine and 6 mg xylazine/kg body weight intraperitoneally, and blood was drawn from the portal vein. Portions of liver tissue were frozen immediately in liquid nitrogen, whereas others were frozen fixed in OCT-mounting media (medite, Burgdorf, Germany) for later sectioning and mounting on microscope slides.
Cell Culture and Treatment
Alpha mouse liver 12 (AML-12) cells obtained from American type culture collection (ATCC, Manassas, USA) were grown in DMEM/F12 media (PAN-Biotech, Aidenbach, Germany) supplemented with 10% fetal bovine serum, 40 ng/ml dexamethasone, 0.005 mg/ml insulin, 5 ng/ml selenium and 0.005 mg/ml transferrin (PAA Laboratories, Colbe, Germany), as well as penicillin and streptomycin at 37°C. At 70% confluence, cells were serum starved for 18 h in starvation media supplemented with bovine serum albumin (BSA; 0.01%), insulin, selenium and transferring, as well as penicillin and streptomycin. Cells were then challenged with 10 ng/ml PAI-1 (Sigma, Steinheim, Germany) for 24 h. In addition, some cells were concomitantly treated with 20 ng/ml hepatocyte growth factor (HGF; Sigma).
Cells were rinsed twice with phosphate-buffered saline (PBS) and either used for triglyceride isolation or measurements of microsomal triglyceride transfer protein (MTTP) activity as detailed below.
Immunoblots
Liver tissue was homogenized in a lysis buffer (20 mmol/l MOPS, 150 mmol/l NaCl, 1 mmol/l EDTA, 1% Nonidet P-40, 1% sodium deoxycholate and 0.1% SDS) containing a protease and phosphatase inhibitors mix (Sigma) to prepare total protein lysates. Cytosolic protein lysates were prepared by homogenizing liver tissue in a lysis buffer (1 mol/l HEPES, 1 mol/l MgCl2, 2 mol/l KCl and 1 mol/L DTT) containing a protease and phosphatase inhibitors mix (Sigma). Proteins (30 or 60 μg protein/well) were separated in 8–15% SDS-polyacrylamide gels depending on the respective molecular weight of the target protein. Proteins were transferred onto Hybond-P polyvinylidene difluoride membranes (Amersham Biosciences, Freiburg, Germany) using a semidry electroblotter. The resulting blots were then probed with antibodies against phospho-c-Met, total c-Met, TNF-α, β-actin, phospho Akt or total Akt (all obtained from Cell Signaling Technology, Danvers, USA). Bands were visualized using the Super Signal Western Dura kit (Thermoscientific, Rockford, USA). To ensure equal loading, all blots were stained with Ponceau red. Protein bands were densitometrically analyzed using the Flurochem Software (Alpha Innotech, San Leandro, USA).
Hepatic Triglyceride Determination and Lipid Staining
Mouse liver tissue was homogenized in 2 × PBS. Tissue lipids were extracted with methanol:chloroform (1:2), dried in an evaporating centrifuge and resuspended in 5% fat-free BSA. Colorimetric assessment of triglyceride levels was carried out using a commercially available kit (Randox, Krefeld, Germany). Values were normalized to protein concentration in liver homogenate using the Bradford assay (Bio-Rad Laboratories, Munich, Germany). Triglyceride levels in cell cultures were determined by using a commercially available kit (Bio Vision, California, USA) following the instructions of the manufacturer. In addition, to determine hepatic lipid accumulation, frozen sections of liver (10 μm) were stained with Oil Red O (Sigma) for 15 min, washed and counterstained with hematoxylin for 45 s (Sigma).
RNA Isolation and Real-Time RT-PCR
Total RNA was extracted from liver tissue samples using Trifast (PEQLAB, Erlangen, Germany). RNA concentrations were determined spectrophotometrically and 1 μg of total RNA was subjected to DNase digestion, followed by a reverse transcription using a Moloney murine leukemia virus reverse transcriptase and oligo (dT) primers (Fermentas, St Leon-Rot, Germany). PCR primers were designed using the software Primer 3 (Whitehead Institute for Biomedical Research, Massachusetts, USA) and are summarized in Table 1. Sybr Green Universal PCR Master Mix (Applied Biosystems, Damstadt, Germany) was used to prepare the PCR mix. Primers were added to a final concentration of 300–400 nmol/l.
The amplification reactions were carried out in an iCycler (Bio-Rad) with initial hold steps (95°C for 2 min) and 50 cycles of a three-step PCR (95°C for 15 s, 60°C for 15 s and 72°C for 30 s). The fluorescence intensity of each sample was measured at each temperature change to monitor amplification of the target gene. The comparative CT method was used to determine the amount of target gene, normalized to an endogenous reference (18S) and relative to a calibrator (2---ΔΔCt).
Clinical Chemistry and Pathologic Evaluation
Plasma alanine aminotransferase (ALT) activity was determined using a commercially available kit (Randox, Krefeld, Germany). Paraffin sections of liver (5 μm) were stained with hematoxylin and eosin to assess liver histology.
Immunohistochemical staining for cluster of differentiation 3-ζ
To determine cluster of differentiation 3-ζ (CD3-ζ), paraffin-embedded liver sections (5 μm) were deparaffinized and rehydrated in ethanol solutions with decreasing concentrations. Liver tissue was stained for CD3-ζ by blocking slides with 8% BSA for 45 min, followed by washing with 2% BSA and an overnight incubation with a polyclonal primary antibody solution (Santa Cruz Biotechnology, Heidelberg, Germany) in a humidified chamber at 4°C. Liver sections were washed, and CD3-ζ was detected by incubating slides with a peroxidase-linked secondary antibody and diaminobenzidine (Peroxidase Envision Kit; DAKO, Hamburg, Germany). A negative control was carried along where PBS tween was added instead of CD3-ζ antibody.
Endotoxin Assay
Endotoxin was determined as described previously in detail.13 Briefly, plasma samples were incubated at 70°C for 20 min. Plasma endotoxin levels were determined using a commercially available endpoint Limulus Amebocyte Lysate assay (Charles River, L’Arbaesle, France) following the instructions of the manufacturer.
MTTP Assay in Liver Tissue and AML-12 Cells
Liver tissue was homogenized in a lysis buffer (1 mmol/l Tris, pH 7.6, 1 mmol/l EGTA, 1 mmol/l MgCl2 and protease inhibitors) and centrifuged at 7400 g for 30 min at 4°C. Protein concentration was determined using the Bradford assay, and 100 μg of protein was used to determine the MTTP activity using a commercially available kit according to the manufacturer's instructions (Chylos, New York, USA). To determine MTTP activity in AML-12 cells, cells were washed with ice-cold PBS, homogenized in a lysis buffer (1 mmol/l Tris, pH 7.6, 1 mmol/l EGTA, 1 mmol/l MgCl2 and protease inhibitors) and then centrifuged at full speed for 1 h at 4°C The supernatant was used to determine the MTTP activity using a commercially available kit according to the manufacturer's instructions (Chylos).
Statistical Analysis
Data are expressed as means+s.e.m. Student's t-test or one-way ANOVA with Tukey's post hoc test were used to determine the statistical differences between the treatment groups as appropriate. A P-value <0.05 was considered to be significant.
RESULTS
PAI-1 and Fructose-Induced Steatosis
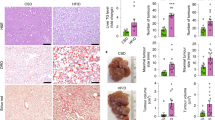
As shown before by our own group,19, 21 chronic intake of fructose led to a significant increase of triglycerides in the livers of wild-type mice (plus ∼5.8-fold in comparison with controls; Figure 1c). This fructose-induced steatosis was associated with a significant ∼2.5-fold increase in PAI-1 mRNA expression in livers of fructose-fed mice in comparison with water controls (see Table 2). To test the hypothesis that PAI-1 is causally involved on the onset of fructose-induced steatosis mice, PAI-1−/− mice were fed with 30% fructose solution or water for 8 weeks. Lipid staining, pathological changes and triglyceride content did not differ between wild-type and PAI-1−/− mice fed with water (Figure 1). Contrary to the findings in fructose-fed wild-type mice, hepatic triglyceride accumulation was only increased by approximately twofold in livers of PAI-1−/− mice exposed to fructose in comparison with PAI-1−/− mice fed with water (Figure 1c). Similar results were also found when staining liver sections with hematoxylin and eosin and oil red O, respectively (Figure 1a and b). In line with these findings the liver to body weight ratio and ALT levels in plasma were also only found to be increased in wild-type animals fed fructose (Table 3). Weight gain was also significantly higher in fructose-fed mice than in water fed or only controls; however, weight gain did not differ between fructose-fed groups (Table 3). Despite the protective effects of the loss of PAI-1 on the onset of fructose-induced hepatic steatosis, both portal endotoxin and hepatic TNF-α protein concentration was significantly increased in fructose-fed mice regardless of strain in comparison with water controls (Figure 2).
Effect of PAI-1 deletion on hepatic lipid accumulation and histology in fructose-fed mice. (a) Representative photomicrographs of hematoxylin and eosin (H & E) staining of liver sections. (b) Representative photomicrographs of oil red O staining of liver sections. (c) Quantitation of hepatic triglycerides content (n=5–7). Data are expressed as means±s.e.m. aP<0.05 compared with wild-type mice fed with plain water. bP<0.05 compared with PAI-1−/− mice fed with plain water. cP<0.05 compared with wild-type (WT) fructose-fed mice.
Effect of PAI-1 deletion on portal endotoxin and hepatic TNF-α protein levels in fructose-fed mice. (a) Endotoxin levels (n=5–6). (b) Representative photographs of western blots of TNF-α and β-actin (c) quantitative analysis of blots (n=5). Data are expressed as means±s.e.m. aP<0.05 compared with wild-type (WT) mice fed with plain water. bP<0.05 compared with PAI-1−/− mice fed with plain water.
Enhanced c-Met Signaling, an Increased Lipoprotein Synthesis and Phosphorylation of Akt and mRNA Expression of B-cell Lymphoma Extra Large are Associated with the Protective Effect of PAI-1 Deletion
As it has been shown before that PAI-1 has a crucial role in the activation of the hepatocyte growth factor-c-Met-depending modulation of hepatic lipid metabolism in hepatic steatosis of various etiologies (eg, alcohol and endotoxin; for overview, see ref. 26), we determined the effect of fructose feeding on c-Met phosphorylation, expression of apolipoprotein B (ApoB) mRNA and activity of MTTP in livers of PAI-1−/− and wild-type mice. No differences in phosphorylation of c-Met were found between livers of water controls regardless of strain and fructose-fed wild-type mice. In contrast, in PAI-1−/− mice fed chronically with 30% fructose solution, phosphorylation was significantly increased in comparison with wild-type controls fed with water (plus approximately 9-fold in comparison with wild-type and plus approximately 4-fold in comparison with PAI-1−/− mice fed with water; Figure 3a). In line with these findings, expression of ApoB mRNA was only found to be increased in fructose-fed PAI-1−/− mice (plus ∼3.1-fold in comparison with wild-type and plus ∼2.3-fold in comparison with PAI-1−/− mice fed with water), whereas those of fructose-fed wild-type mice remained at the level of controls (Figure 3b). Furthermore, MTTP activity was also found to be increased only in PAI-1−/− mice fed with fructose (30% in comparison with wild-type mice (P<0.05) and 10% in comparison with PAI-1−/− mice fed with water (P is not significant; Figure 3c)).
Effect of PAI-1 deletion on c-Met, ApoB mRNA expression and MTTP activity, as well as Akt protein levels in livers of fructose-fed mice. (a) Representative photographs of western blots of phospho-c-Met, total c-Met and quantitative analysis of blots (n=4) (b) ApoB mRNA levels normalized to β-actin expression (n=5) (c) MTTP activity (n=5) and (d) representative photographs of western blots of phospho Akt and total Akt, and quantitative analysis of blots (n=5). Data are expressed as means±s.e.m. aP<0.05 compared with wild-type (WT) mice fed with plain water. bP<0.05 compared with PAI-1−/− mice fed with plain water. cP<0.05 compared with wild-type fructose-fed mice.
As it has been shown before by others that c-Met might also be involved in the regulation of Akt,31, 32 we determined Akt phosphorylation in livers of fructose and water-fed mice. Despite elevated TNF-α protein levels in both fructose-fed groups, phosphorylation levels of Akt were only found to be decreased in livers of fructose-fed wild-type mice (Figure 3d). In livers of fructose-fed PAI-1−/− mice, phosphorylation of Akt did not differ from that of water-fed controls (Figure 3d). In line with these findings, mRNA expression of B-cell lymphoma extra large (BCL-XL), which was shown to be regulated through HGF-c-Met-Akt-dependent pathways,33 was found to be significantly reduced in livers of wild-type mice fed with fructose. In contrast, in livers of PAI-1−/− mice fed with fructose, expression of BCL-XL was significantly higher than in livers of PAI-1−/− mice fed with water (Table 3).
Effect of PAI-1 and HGF on Fat Accumulation and MTTP Activity in AML-12 Cells
To further investigate the role of HGF and PAI-1 in modulating triglyceride export in the liver AML-12 cells, a model of murine hepatocytes was challenged with PAI-1 in the presence or absence of HGF. The treatment of AML-12 cells with HGF had no effect on triglyceride content and MTTP activity (Figure 4a and b). In contrast, when the cells were challenged with PAI-1, MTTP activity was significantly lower in comparison with naïve cells (∼81%). In line with these findings, triglyceride concentration was significantly increased in these cells (approximately +10-fold in comparison with naïve cells) too. Interestingly, when cells were treated with HGF while concomitantly being challenged with PAI-1, MTTP activity remained at the level of naïve cells and cells did not accumulate triglycerides (Figure 4a and b).
Effect of PAI-1 Deletion on Hepatic Natural Killer T Cells
As it has been suggested by the results of other groups that MTTP regulates endogenous and exogenous antigen presentation by group 1 cluster of differentiation 1d (CD1d) molecules34 and also its function (eg, binding and activation of iNKT cells),35 we determined expression of CD1d, markers of iNKT cells, as well as iNKT cell activation in livers of PAI-1−/− and wild-type mice fed with water or fructose. Expression of CD1d was markedly higher in livers of PAI-1−/− mice in comparison with wild-type mice regardless of additional treatment; however, as expression varied considerably between animals in the water control group, differences did only reach a level of significance for fructose-fed PAI-1−/− mice (P<0.05 in comparison with wild-type controls and fructose-fed wild types; Table 2). In line with these findings, expressions of the iNKT cell markers CD3-ɛ and natural killer 1.1 (NK1.1) were also found to be significantly higher in livers of PAI-1−/− mice in comparison with wild-type mice regardless of additional treatments (Figure 5b and c). Similar results were also found for CD3-ζ staining in liver sections of PAI-1−/− mice regardless of treatment (representative pictures of staining are shown in Figure 5a). Interestingly, markers of iNKT cell activation (eg, interleukin 15 (IL-15) and interferon-γ (IFNγ)) were both only found to be significantly induced in livers of PAI-1−/− mice fed with fructose, whereas expression levels of IL-15 and IFN-γ did not differ between water controls regardless of the strain and wild-type mice fed with fructose (Table 2).
Effect of PAI-1 deletion on NKT cell markers in livers of fructose-fed mice. (a) Representative photographs of CD3-ζ staining of liver sections; brown stained parts marked with arrows indicate CD3-ζ-positive cells. (b) CD3-ɛ mRNA levels normalized to 18S expression (n=5). (c) NK1.1 mRNA levels normalized to 18S expression (n=5). Data are expressed as means±s.e.m. aP<0.05 compared with wild-type (WT) mice fed with plain water. bP<0.05 compared with PAI-1−/− mice fed with plain water. cP<0.05 compared with wild-type fructose-fed mice.
DISCUSSION
Results of several human and animal studies suggest that an increased dietary fructose intake is a risk factor in the development of NAFLD.13, 14, 16, 36 In line with these findings, results of pilot human studies suggest that a reduction of fructose intake may exert beneficial effects on the progression of NAFLD.14 We recently reported that the onset of fructose-induced hepatic steatosis is associated with markedly increased endotoxin levels in portal plasma, an increased formation of ROS and an induction of nuclear factor-κB activity as well as TNF-α mRNA expression in the liver of mice.19 In humans with NAFLD and also in livers of obese mice (eg, ob/ob) with NAFLD, PAI-1 expression was reported to be increased, too.13, 28 We further reported that in TNFR1−/− mice, the onset of fructose-induced liver steatosis is markedly attenuated and the loss of TNFR1 in these mice was associated with normalization of PAI-1 expression in the liver.25 However, whether PAI-1 is causally involved in mediating the effects of fructose on the liver has not yet been clarified. In the present study, the hypothesis that PAI-1 is critically involved in the onset of fructose-induced NAFLD was tested in a mouse model. Indeed, in livers of fructose-fed mice, PAI-1 expression was induced. Furthermore, despite similarly elevated endotoxin levels in portal plasma and TNF-α protein concentrations in the liver, fat accumulation in livers of PAI-1−/− mice resulting from chronic exposure to 30% fructose solution was markedly attenuated (minus ∼50%). Interestingly, the magnitude of protection against fructose-induced hepatic steatosis found in livers of PAI-1−/− mice fed with fructose was similar to that reported before by our group for livers of fructose-fed TLR-4-mutant mice and mice treated with non-resorbable antibiotics, as well as TNFR1−/− mice.19, 21, 25 Taken together, these data further suggest that PAI-1 has a casual role on the onset of NAFLD in the liver. These data also suggest that PAI-1 may be a critical factor in mediating the effect of the increased translocation of bacterial endotoxin and the subsequent activation of TLR-4-dependent signaling cascades found in livers of mice chronically exposed to fructose.
Results of studies using animal models of acute and chronic alcohol-induced liver damage have suggested that PAI-1 may modulate hepatic lipid export.30 Indeed, PAI-1 is an inhibitor of urokinase-type plasminogen activator (uPA), which has been shown before to be involved not only in fibrinolysis but also to activate prohepatocyte growth factor (pro-HGF) to HGF.37, 38 Results of in vitro studies have suggested that HGF can stimulate the MTTP-dependent lipoprotein secretion in hepatocytes through binding to its receptor c-Met.39 In support of these in vitro results, it has been shown before in livers of mice after acute alcohol treatment that c-Met phosphorylation and MTTP activity, as well as ApoB concentration were upregulated in PAI-1−/− and TNFR1−/− mice.30 Furthermore, it has been shown that blocking lipoprotein secretion in the liver may increase the susceptibility of the liver to certain toxin challenges (eg, endotoxin).40 In the present study, phosphorylation of c-Met as well as mRNA expression of ApoB and MTTP activity were markedly upregulated in livers of PAI-1−/− mice fed with fructose, whereas in wild-type mice fed with fructose, phosphorylation of c-Met, ApoB mRNA expression and MTTP activity remained at the level of controls. Furthermore, using a murine cell culture model of hepatocytes (eg, AML-12 cells), we were able to show that the challenging of hepatocytes with PAI-1 may result in a reduction of MTTP activity and accumulation of triglyceride in cells, whereas this effect of PAI-1 was totally abolished in the presence of HGF. Taken together, these data suggest that in the present study, the protective effect of the loss of PAI-1 may have resulted from a compensatory increase in very-low-density lipoprotein (VLDL) synthesized to excrete the greater amount of triglyceride synthesis in hepatocytes resulting from the metabolism of fructose. These results further suggest that PAI-1 through an inhibition of uPA may modulate the activation of pro-HGF to HGF. This lack of active HGF may in turn lead to a suppression of the MTTP activity, resulting in an accumulation of triglycerides that are no longer shuttled out of the cells via ApoB/VLDL.
It has been reported before by others that c-Met may be involved in the regulation of phosphoinositide 3-kinase (PI3K)/Akt signaling cascade.31, 32 In line with these findings, phosphorylation of Akt was only found to be altered in fructose-fed wild-type mice. Indeed, expression of antiapoptotic BCL-2 family protein BCL-XL, which was shown33 to be modulated by HGF-c-Met-Akt-dependent pathways, was induced in livers of fructose-fed PAI-1−/− mice, whereas a similar effect was not found in livers of fructose-fed wild-type mice. These findings are also supported by the results of in vitro cell culture models of other groups in which it was shown that PAI-1 may act as a regulator of cell survival through altering the PI3K/Akt pathway.41 Taken together, these results suggest that the loss of PAI-1 probably through c-Met-dependent signaling pathway may protect the liver from impaired PI3K/Akt signaling associated with the chronic intake of fructose and may even influence apoptosis found under these conditions. Exact mechanisms involved in this rescue of PI3K/ Akt signaling and their implications on apoptosis, as well as the liver and the development of NAFLD remain to be determined.
It has been suggested by the results of Dougan et al35 and Brozovic et al42 that the MTTP is a critical factor not only for the lipidation of ApoB and hence the generation of VLDL but also in the regulation of CD1d function. Indeed, in an in vitro reductionist system, MTTP was able to transfer phospholipids to CD1d,35, 43 further suggesting that MTTP lipidation of CD1d is critical for CD1d to present both endogenous (ER loaded) and exogenous (endosomal or surface loaded) antigens to CD1d-restricted NKT cells. It has further been suggested by the results of Dougan et al43 that MTTP activity is required for NKT cell activation and NKT cell development. Interestingly, it was also shown by other groups that MTTP inhibition can not only cause a modest decrease in surface CD1d but also a marked defect in CD1d antigen presentation.35, 42 In addition, in different mouse models of NAFLD (eg, ob/ob mice and diet-induced NAFLD), it has been shown that the development of the disease was associated with a markedly decreased number of CD1d-reactive NKT cells in the liver.44, 45, 46 Furthermore, it was recently reported by Kremer et al47 that in hepatosteatosis, number of hepatic NKT cells is reduced in a Kupffer cell-dependent manner. In the present study, expression of CD1d and markers of NKT cells (eg, CD3-ɛ and NK1.1) were markedly higher in livers of PAI-1−/− mice regardless of feeding in comparison with wild-type mice. However, when determining IL-15 and IFN-γ, both shown before by others to be indicative for NKT cell activation,48, 49 we found a significant induction of these cytokines only in livers of fructose-fed PAI-1−/− mice. Taken together, these data suggest that in line with earlier findings of other groups,35, 42 the increased activity of MTTP found in livers of PAI-1−/− mice was associated not only with an increase of CD1d-reactive NKT cells but also an activation of these cells. Furthermore, these data also suggest that the loss of PAI-1 may be associated with an increased release of cytokines such as IL-15 and IFN-γ from NKT cells, adding to the protective effects found in the mice. Indeed, it has been suggested by Suzuki et al50 that IL-15 may contribute to regeneration activity in damaged livers. However, further studies are necessary to determine the role of NKT cells and IL-15 on the onset of fructose-induced steatosis.
The results of the present study suggest that PAI-1 may be a critical factor on the onset of fructose-induced steatosis through mechanisms involving a suppression of the compensatory upregulation of lipid export necessary to protect the liver from the increased amount of lipids resulting from a chronic dietary intake of fructose (see Figure 6). In addition, our data suggest that PAI-1 may also be involved in modulating CD1-reactive NKT cells in the liver (see Figure 6). However, whether similar mechanisms are also involved in the development of NAFLD in humans remains to be determined.
Possible molecular mechanism involved in the development of fructose-induced fatty liver. Chronic intake of fructose may lead to elevated bacterial endotoxin levels in the portal blood and an activation of Kupffer cells, subsequently leading to an increased formation of ROS and an nuclear factor-κB-dependent induction of TNF-α. TNF-α can then cause insulin resistance (IR) in hepatocytes and an induction of PAI-1, which in turn may, through the c-Met signaling pathway, modulate hepatic lipid export, CD1d lipidation and NKT cell activation.
References
Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–923.
Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among US adolescents using the definition from the International Diabetes Federation. Diabetes Care 2008;31:587–589.
Eckel RH, Grundy SM, Zimmet PZ . The metabolic syndrome. Lancet 2005;365:1415–1428.
Zimmet P, Magliano D, Matsuzawa Y, et al. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb 2005;12:295–300.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–1419.
Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995;22:1714–1719.
Fassio E, Alvarez E, Dominguez N, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004;40:820–826.
Adams LA, Lymp JF, St SJ, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121.
Brunt EM . Nonalcoholic steatohepatitis. Semin Liver Dis 2004;24:3–20.
US Department of Agriculture. MyPyramid.gov Website. Washington, DC. http://www.cnpp.usda.gov/Publications/MyPyramid/OriginalFoodGuidePyramids/FGP/FGPPamphlet.pdf (accessed 1992).
Hanover LM, White JS . Manufacturing, composition, and applications of fructose. Am J Clin Nutr 1993;58:724S–732S.
Forshee RA, Storey ML, Allison DB, et al. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr 2007;47:561–582.
Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138:1452–1455.
Assy N, Nasser G, Kamayse I, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–816.
Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 2007;47:711–717.
Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–999.
Ackerman Z, Oron-Herman M, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 2005;45:1012–1018.
Armutcu F, Coskun O, Gurel A, et al. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem 2005;38:540–547.
Bergheim I, Weber S, Vos M, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008;48:983–992.
Jurgens H, Haass W, Castaneda TR, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res 2005;13:1146–1156.
Spruss A, Kanuri G, Wagnerberger S, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094–1104.
Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 2006;55:415–424.
Ma X, Hua J, Li Z . Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol 2008;49:821–830.
Kudo H, Takahara T, Yata Y, et al. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol 2009;51:168–175.
Kanuri G, Spruss A, Wagnerberger S, et al. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem; published online 27 August 2010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20801629.
Arteel GE . New role of plasminogen activator inhibitor-1 in alcohol-induced liver injury. J Gastroenterol Hepatol 2008;23:S54–S59.
Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16:1394–1399.
Alessi MC, Bastelica D, Mavri A, et al. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol 2003;23:1262–1268.
Ma LJ, Mao SL, Taylor KL, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 2004;53:336–346.
Bergheim I, Guo L, Davis MA, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology 2006;130:2099–2112.
Ye M, Hu D, Tu L, et al. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest Ophthalmol Vis Sci 2008;49:497–504.
Taher TE, Tjin EP, Beuling EA, et al. c-Cbl is involved in Met signaling in B cells and mediates hepatocyte growth factor-induced receptor ubiquitination. J Immunol 2002;169:3793–3800.
Suzuki H, Toyoda M, Horiguchi N, et al. Hepatocyte growth factor protects against Fas-mediated liver apoptosis in transgenic mice. Liver Int 2009;29:1562–1568.
Kaser A, Hava DL, Dougan SK, et al. Microsomal triglyceride transfer protein regulates endogenous and exogenous antigen presentation by group 1 CD1 molecules. Eur J Immunol 2008;38:2351–2359.
Dougan SK, Salas A, Rava P, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med 2005;202:529–539.
Solga S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci 2004;49:1578–1583.
Naldini L, Vigna E, Bardelli A, et al. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem 1995;270:603–611.
Taniyama Y, Morishita R, Nakagami H, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation 2000;102:246–252.
Kaibori M, Kwon AH, Oda M, et al. Hepatocyte growth factor stimulates synthesis of lipids and secretion of lipoproteins in rat hepatocytes. Hepatology 1998;27:1354–1361.
Bjorkegren J, Beigneux A, Bergo MO, et al. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J Biol Chem 2002;277:5476–5483.
Balsara RD, Castellino FJ, Ploplis VA . A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. J Biol Chem 2006;281:22527–22536.
Brozovic S, Nagaishi T, Yoshida M, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med 2004;10:535–539.
Dougan SK, Rava P, Hussain MM, et al. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med 2007;204:533–545.
Li Z, Soloski MJ, Diehl AM . Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology 2005;42:880–885.
Li Z, Oben JA, Yang S, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology 2004;40:434–441.
Yang L, Jhaveri R, Huang J, et al. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest 2007;87:927–937.
Kremer M, Thomas E, Milton RJ, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology 2010;51:130–141.
Li B, Sun R, Wei H, et al. Interleukin-15 prevents concanavalin A-induced liver injury in mice via NKT cell-dependent mechanism. Hepatology 2006;43:1211–1219.
Olson Jr CM, Bates TC, Izadi H, et al. Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J Immunol 2009;182:3728–3734.
Suzuki A, McCall S, Choi SS, et al. Interleukin-15 increases hepatic regenerative activity. J Hepatol 2006;45:410–418.
Acknowledgements
This study was supported by the German Research Foundation (Grant no. BE 2376/4-1 (IB)) and BMBF (Grant no. 03105084(IB)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Plasminogen activator inhibitor-1-/- mice are protected from fructose-induced liver steatosis. The protective effect is associated with an induction of the HGF/c-Met-signaling cascade and activation of the microsomal triglyceride transfer protein in natural killer T-cells.
Rights and permissions
About this article
Cite this article
Kanuri, G., Spruss, A., Wagnerberger, S. et al. Fructose-induced steatosis in mice: role of plasminogen activator inhibitor-1, microsomal triglyceride transfer protein and NKT cells. Lab Invest 91, 885–895 (2011). https://doi.org/10.1038/labinvest.2011.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2011.44
Keywords
This article is cited by
-
Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway
Scientific Reports (2021)
-
A new method to induce nonalcoholic steatohepatitis (NASH) in mice
BMC Gastroenterology (2019)
-
Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD)
Archives of Toxicology (2017)
-
Role of PAI-1 in Pediatric Obesity and Nonalcoholic Fatty Liver Disease
Current Cardiovascular Risk Reports (2017)
-
Plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, e-selectin and C-reactive protein levels in response to 4-week very-high-fructose or -glucose diets
European Journal of Clinical Nutrition (2014)