Abstract
Metastatic spread of tumor cells to vital organs is the major cause of mortality in cancer patients. Bcl-2, a key antiapoptotic protein, is expressed at high levels in a number of human tumors. We have recently shown that Bcl-2 is also overexpressed in tumor-associated blood vessels in head-and-neck cancer patients. Interestingly, enhanced Bcl-2 expression in tumor blood vessels is directly correlated with metastatic status of these cancer patients. In addition, endothelial cells (ECs) expressing Bcl-2 showed increased production of interleukin-8 (IL-8) resulting in significantly enhanced tumor cell proliferation and tumor cell invasion. Therefore, we hypothesized that Bcl-2 expression in tumor-associated ECs may promote tumor metastasis by enhancing tumor cell invasiveness and release in the circulation. To test our hypothesis, we coimplanted tumor cells along with ECs expressing Bcl-2 (EC-Bcl-2) in the flanks of SCID mice. Our results demonstrate that incorporation of EC-Bcl-2 in primary tumors significantly enhanced tumor cell metastasis to lungs and this EC-Bcl-2-mediated tumor metastasis was independent of primary tumor size. In addition, Bcl-2-mediated tumor metastasis directly correlated with increased tumor angiogenesis. Bcl-2 expression in ECs also promoted transendothelial cell permeability, blood vessel leakiness and tumor cell invasion. EC-Bcl-2-mediated tumor cell proliferation and tumor cell invasion were significantly mediated by IL-8. These results suggest that Bcl-2, when expressed at higher levels in tumor-associated ECs, may promote tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor cell invasiveness.
Similar content being viewed by others
Main
The metastatic spread of solid tumors is directly or indirectly responsible for more than 90% of cancer mortalities.1 Our understanding of the molecular and biological events that contribute to tumor cell progression has increased considerably over the last decade.2 However, the prognosis for patients who are diagnosed with advanced invasive or metastatic disease is not much better than it was decades ago.3 The process of metastasis is highly complex and involves close cooperation between cancer cells and accessory cells that comprise the tumor microenvironment. Tumor-associated endothelial cells (ECs) are an important component of tumor microenvironment. They not only promote tumor growth but also play an important role in tumor metastasis.4, 5, 6 A number of studies have shown a direct correlation between vascular density in the primary tumor and tumor metastasis to distal sites for patients with cancers of the head-and-neck,7 breast,8 prostate,9 lung,10 stomach11 and cervix.12 Angiogenesis is thought to facilitate tumor metastasis by providing an increased density of immature, highly permeable blood vessels that have little basement membrane and fewer intercellular junctional complexes than normal mature vessels.13 Unlike their normal counter parts, tumor blood vessels typically have irregular diameters and branching patterns14 and are abnormally leaky.15, 16, 17
The Bcl-2 family of proteins are key regulators of apoptosis.18 The Bcl-2 family is subdivided into two classes, antiapoptotic members such as Bcl-2 and Bcl-xL, and proapoptotic members, Bax and Bak.19 Recently, we and others have shown that Bcl-2, in addition to regulating cytochrome c release from mitochondria, can also mediate a signaling cascade that renders ECs resistant to a number of anticancer agents.20, 21 Bcl-2 has been shown to bind to the multifunctional chaperone protein BAG-1.22 In turn, BAG-1 binds to and activates the protein kinase Raf-1.21 Raf-1 kinase plays a central role in the conserved Ras/Raf/MEK/ERK pathway, acting to relay signals from activated Ras proteins through MAPK/ERK kinase 1/2 (MEK1/2) to ERK1/2.23 ERK can phosphorylate a number of downstream targets including Rsk, Elk1, cFos and various transcription factors24 leading to enhanced expression of a number of proteins including survivin.25 We have previously shown that VEGF, a key angiogenic factor, upregulates the expression of Bcl-226 and ECs expressing Bcl-2 in turn promote tumor progression.27 However, little is known about the role and mechanism by which endothelial Bcl-2 might enhance tumor metastasis.
In this study, we have examined whether Bcl-2 expression in tumor-associated ECs could promote tumor cells metastasis to distal sites. Here, we report that Bcl-2 expression in tumor-associated ECs is associated with a markedly higher incidence of squamous carcinoma metastasis to lungs. This EC-Bcl-2-mediated tumor metastasis was independent of primary tumor size and directly correlated with enhanced tumor angiogenesis and blood vessel leakiness in primary tumors. In addition, interleukin-8 (IL-8) production by Bcl-2-expressing ECs promoted tumor cell proliferation and tumor cell invasion.
MATERIALS AND METHODS
Cell Cultures
Primary human dermal microvascular ECs were purchased from Biowhittaker (Walkersville, MD). ECs were maintained in endothelial cell basal medium-2 (EBM-2) containing 5% FBS and growth supplements. Oral squamous carcinoma cell line (OSCC-3) and UM-SCC-74A (a squamous carcinoma cell line derived from the base of tongue) were a kind gift from M Lingen (University of Chicago) and T Carey (University of Michigan), respectably. Both OSCC-3 and UM-SCC-74A cells were maintained in Dulbecco's modified eagle medium (DMEM) supplemented with 10% FBS.
Transduction of Endothelial Cells with Bcl-2 and Tumor Cells with GFP
Bcl-2 was introduced into human microvascular ECs as described previously.27 The Bcl-2 construct or the vector alone was introduced into PA317 amphotropic packing cells with lipofectin. Viral supernatants were collected after 24 h, centrifuged, filtered and stored at −70°C. ECs were transduced with either Bcl-2 (EC-Bcl-2) or control vector (EC-VC) by overnight incubation with one-tenth dilution of the viral supernatant in the presence of 6 μg/ml polybrene. Similarly, tumor cells (OSCC-3 and UM-SCC-74A) were transduced with GFP gene as described above using retroviral vector (obtained from University Michigan Vector Core). Transfected tumor cells (OSCC-3-GFP and UM-SCC-74A-GFP) were selected by treating them with G418 (200 μg/ml) for 1 week. Tumor cells expressing high levels of GFP were further selected by cell sorting using flow cytometry. Bcl-2 expression in ECs was confirmed by northern and western blot analysis. GFP expression in tumor cells was analyzed by examining the cells using fluorescent phase contrast microscope.
Immunofluorescence Staining
Tissue microarrays (TMA) prepared from human head-and-neck squamous cell carcinoma (HNSCC) samples were stained with Bcl-2 and factor VIII to analyze the expression of Bcl-2 in tumor microvasculature as described before.20 The TMA-contained tissue samples from a total of 102 patients with different clinical and pathological stages. At least three cores (0.6 mm diameter) from tumor samples were represented from each patient. The TMA covered a whole spectrum of HNSCC including normal internal controls, primary SCC from lymph node-negative patients and primary SCC from lymph node-positive patients. Sections cut from the TMA were deparaffinized and antigen retrieval was performed by incubating the slides at 92°C for 20 min in target retrieval solution (Dako). Nonspecific sites in the TMAs were blocked by incubation in nonserum protein block solution (Dako) for 10 min at room temperature followed by incubation with mouse antihuman Bcl-2 antibody (ready to use, Dako) and rabbit antihuman factor VIII antibody (1:60, Dako) for 1 h at room temperature. The sections were washed with PBS and incubated with antimouse FITC (1:50, Sigma) and antirabbit rhodamine (1:50, Sigma) for 30 min at room temperature. Slides were washed and mounted in aqua mount (Polyscience Inc.). Separate pictures of each tissue sample for factor VIII and Bcl-2 were taken and then superimposed to quantify the Bcl-2-positive vessels.
Tumor Development In Vivo
Tumors grown in the flanks of SCID mice were used to investigate the effect of Bcl-2 expression on tumor growth, tumor angiogenesis and tumor metastasis. Tumor cells (OSCC-3-GFP or UM-SCC-74A-GFP, 0.5 × 106) and ECs (EC-Bcl-2 or EC-VC 0.5 × 106) were mixed with 100 μl of matrigel and injected in the flanks of SCID mice. Tumor volume measurements began on day 3 and continued twice a week until the end of the study. The length and width were measured using a digital caliper and tumor volumes were calculated using the formula, volume (mm3)=L × W2/2 (length, L, mm; width W, mm). After 3 weeks, primary tumors and lungs were carefully removed and analyzed for tumor growth, tumor angiogenesis and tumor metastasis to lungs. In a separate study, tumor cells and ECs (EC-Bcl-2 or EC-VC) were coimplanted in SCID mice as described above. Once the tumors reached 200 mm3, tumors were surgically removed and tumor metastasis to lungs was analyzed after 2 weeks.
Quantitaion of Angiogenesis by Immunolocalization of Von Willebrand Factor
Tissue sections were deparaffinized and antigen retrieval was achieved by pressure cooking in Decloaking chamber (Biocare Medical, Walnut Creek, CA) at 120°C for 20 min.28 Tissue sections were then treated with peroxide block solution for 5 min at room temperature followed by 1 h of incubation with primary antibody (anti-Von Willebrand Factor, Dako) at room temperature. Slides were further incubated for 30 min with HRP labeled polymer (Dako EnVision+ system kit) and developed with AEC+ staining kit (Dako). Microvessel density was calculated by counting five random high power fields ( × 200).
Analysis of Tumor Metastasis to Lungs
Lungs from SCID mice were carefully removed on day 21 and cut into two equal parts. One half of each lung was fixed and paraffin embedded for immunohistochemical analysis. The other half of the lung was used to harvest tumor cells. Lungs were finely minced by scissors, washed with sterile serum-free media (DMEM) and treated with collagenase (2.5 mg/ml) for 3 h at 37°C with intermittent shaking. After collagenase treatment, cells were treated with G418 (200 μg/ml) in 100 mm culture dishes to select tumor cells (OSCC-3-GFP and UM-SCC-74A-GFP). After 1 week, tumor cell colonies were counted using phase contrast microscope ( × 50).
Tumor Cell Proliferation Assay
We performed coculture experiments to examine whether Bcl-2-expressing ECs could induce tumor cell proliferation in a paracrine manner. In brief, 3 × 104 tumor cells (OSCC-3 or UM-SCC-74A) were cultured in 24-well plates. Separately, 3 × 104 ECs (EC-Bcl-2 or EC-VC) were plated on 24-well plate inserts and these inserts were carefully layered on top of 24-well plates containing tumor cells. After 72 h, inserts were removed and tumor cells were trypsinized and counted using hemocytometer. For IL-8 neutralization experiments, neutralizing antihuman IL-8 mouse monoclonal antibody (2 μg/ml, R&D systems) was added to lower wells at the start of coculture.
Transendothelial Cell Permeability Assay
Transendothelial cell permeability assay was performed as described previously.29 In brief, EC-Bcl-2 (1 × 105) or EC-VC were cultured in complete EGM (endothelial growth medium) on top of 24-well transwell inserts coated with type 1 collagen (Cohesion, Palo Alto, CA) to form a uniform layer. The transwell inserts containing endothelial cell monolayer were incubated in reduced serum (1%) medium for 1 h before adding FITC dextran (1 mg/ml, MW 40 000) in the presence or absence of VEGF (rhVEGF-A, 50 ng/ml). At 15, 30 min, 1, 2 and 3 h time points, 100 μl of supernatant was carefully removed from the bottom well and replaced with fresh media. The presence of FITC dextran in upper and lower wells was determined with a fluorometer, using an excitation wavelength of 492 nm, and detecting emission of 520 nm. Each experiment was performed in triplicate and three independent assays were carried out for each group.
Blood Vessel Leakiness Model
We analyzed blood vessel leakiness in both normal blood vessels populated with Bcl-2-positive ECs as well as tumor microvessels containing Bcl-2-expressing ECs. Blood vessel leakiness was studied using a protocol previously described by Enis et al.30 In brief, EC-Bcl-2 or EC-VC (2 × 106) were mixed with 500 μl of matrigel and implanted in the right and left flank of SCID mice, respectively. After 10 days, 200 μl of FITC-dextran solution (25 mg/ml) was injected through the tail vein. After 30 min, matrigel plugs were carefully retrieved, fixed and frozen in OCT. Cryosections were analyzed by fluorescence microscope. For tumor blood vessel leakiness, animals were injected with 200 μl of FITC-dextran solution on day 14 and tumor samples were analyzed as described above for matrigel plugs.
Tumor Cell Invasion Assays
The role of Bcl-2 in tumor cell invasion was investigated using a matrigel invasion assay. EC-Bcl-2 or EC-VC (50 000 cells/well) was cultured in 24-well plates for 24 h at 37°C. Separately, 24-well plate inserts (8 μM pore size, Falcon) were coated with 20 μl of matrigel and incubated at 37°C for 30 min to let the matrigel polymerize. Next, 50 000 tumor cells (OSCC-3 or UM-SCC-74A) were carefully layered on top of the matrigel and the inserts were placed in the 24-well plates containing ECs. For IL-8 neutralization experiments, neutralizing antihuman IL-8 mouse monoclonal antibody (2 μg/ml, R&D systems) was added to lower wells at the start of coculture. The plates were further incubated for 24 h at 37°C and the noninvaded cells were carefully removed with a cotton swab. The inserts were then stained with Diff-quick solution II and mounted on glass slides. The number of cells that had invaded through the matrigel was counted in five high power fields.
Statistical Analysis
Data from all the experiments are expressed as mean±s.e.m. Statistical differences were determined by two-way analysis of variance and Student's t-test. A P-value of <0.05 was considered significant.
RESULTS
Tumor Samples from Lymph Node-positive Head-and-neck Cancer Patients Show Significantly Higher Bcl-2-positive Blood Vessels than Lymph Node-negative Patients
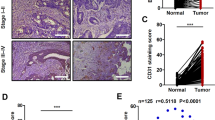
We have previously shown that the upregulation of Bcl-2 in tumor-associated ECs is sufficient to enhance tumor progression in vivo.27 We have also recently shown that Bcl-2 expression is significantly elevated in tumor blood vessels from cancer patients as compared to matched control samples.20 In this study, we compared Bcl-2 expression levels in tumor blood vessels from lymph node-positive cancer patients with lymph node-negative patients. Our results show that tumor samples from lymph node-positive cancer patients had significantly higher number of Bcl-2-positive blood vessels as compared to tumor samples from lymph node-negative patients (Figure 1a). Among the different head-and-neck tumor types, tumor samples from the oral cavity showed the highest expression of Bcl-2 in tumor-associated blood vessels followed by tumor samples from oropharynx, hypopharynx and others that included glottic and supraglottic tumors (Figure 1b). Tumor samples from advanced stage III and IV exhibited markedly higher Bcl-2 expression in tumor blood vessels as compared to tumor samples from stage I or II (Figure 1c). Interestingly, even small tumors from lymph node-positive stage II patients showed significantly higher Bcl-2 expression in tumor blood vessels as compared to lymph node-negative stage II patients. These results, therefore, suggest that enhanced expression of Bcl-2 in tumor blood vessels may promote tumor metastasis.
Bcl-2 expression is highly elevated in tumor samples from lymph node-positive head-and-neck cancer patients. Tissue microarrays (TMA) containing primary tumor samples from head-and-neck cancer patients were double stained with rabbit anti-factor VIII (Rhodamine) and mouse anti-Bcl-2 (FITC) antibodies. The results are expressed as % of vessels positive for Bcl-2+s.e. (a) Percentage of Bcl-2-positive blood vessels in lymph node-negative (n=33), lymph node-positive (n=69) and matched normal control (n=99) samples. (b) Percentage of Bcl-2-positive blood vessels in primary tumor samples from different tumor sites, oral cavity (OC, n=50), oropharynx (OP, n=36), hypopharynx (HP, n=5) and others (glottic and supraglottic, n=11). (c) Percentage of Bcl-2-positive blood vessels in tumor stage I (n=9), II (n=25), III (n=29) and IV (n=39). *Represents a significant difference (P<0.05) in Bcl-2-positive blood vessels.
EC-Bcl-2 Incorporation Along with Tumor Cells Markedly Enhanced Tumor Growth and Tumor Angiogenesis
We used SCID mouse model to investigate the role of EC Bcl-2 expression on tumor growth and tumor angiogenesis. Tumor growth was analyzed by measuring tumor volume twice a week starting at day 3. OSCC-3 tumors containing Bcl-2-expressing ECs (EC-Bcl-2) showed markedly higher tumor growth (Figure 2a1) as compared to OSCC-3 tumor containing ECs with vector alone (EC-VC), and the difference in tumor growth was statistically significant at days 15, 18 and 21. In addition, OSCC-3 tumors containing EC-Bcl-2 showed significantly higher tumor weights at the end of study as compared to OSCC-3 tumors containing EC-VC (Figure 2a2–3). We then used a second squamous cell carcinoma line (UM-SCC-74A) to corroborate our results with OSCC-3 cells. We observed a similar pattern of tumor growth with UM-SCC-74A cells. UM-SCC-74A tumors populated with EC-Bcl-2 cells showed significantly higher tumor volume and tumor weight as compared to UM-SCC-74A tumors containing EC-VC (Figure 2b). To further examine whether Bcl-2 expression in EC promotes tumor cell proliferation in a paracrine manner, we performed coculture experiments. Tumor cells (OSCC-3 and UM-SCC-74A) showed significantly higher proliferation when cocultured with EC-Bcl-2 cells as compared to EC-VC (Figure 3c and d). We have previously shown that EC-Bcl-2 cells produce significantly higher levels of IL-8.27 Therefore, we next examined whether EC-Bcl-2-mediated tumor cell proliferation is mediated through IL-8 in a paracrine manner. Neutralization of IL-8 by anti-IL-8 antibody resulted in partial but significant inhibition of EC-Bcl-2-mediated tumor cell growth (Figure 3c and d). Similar to tumor growth, tumors populated with EC-Bcl-2 also showed significantly higher blood vessel density as compared to tumors containing EC-VC (Figure 3a–b).
Endothelial cells (ECs) expressing Bcl-2 promote tumor growth. Tumor cells (OSCC-3-GFP or UM-SCC-74A-GFP, 0.5 × 106) and ECs (EC-Bcl-2 or EC-VC 0.5 × 106) were mixed with 100 μl of matrigel and injected in the flanks of SCID mice. Tumor volume measurements began on day 3 and continued twice a week until the end of the study. Length and width were measured using a digital caliper and tumor volumes were calculated using the formula, volume (mm3)=L × W2/2 (length, L, mm; width W, mm). At day 21, animals were euthanized and tumors were carefully removed and weighted using digital weighing machine. (a1 and b1) Tumor progression curves for OSCC-3 and UM-SCC-74A tumor, respectively. (a2 and b2) Representative photographs for OSCC-3 and UM-SCC-74A tumor, respectively. (a3 and b3) Tumor weights for OSCC-3 and UM-SCC-74A tumor, respectively. *Represents a significant difference (P<0.05) as compared to the control group.
Bcl-2 expression in endothelial cells (ECs) leads to enhanced tumor angiogenesis and tumor cell proliferation. (a and b) Paraffin-embedded tumor sections were stained for tumor blood vessels using antihuman Von Willebrand factor antibodies. Microvessel density in the tumor samples was calculated by counting six random fields ( × 20). (a) Microvessel density levels in OSCC-3+EC-VC and OSCC-3+EC-Bcl-2 tumor samples. (b) Microvessel density levels in UM-SCC-74A+EC-VC and UM-SCC-74A+EC-Bcl-2 tumor samples. (c and d) EC-Bcl-2-mediated tumor cell proliferation was analyzed using coculture assay. Tumor cells (c; OSCC-3 or d; UM-SCC-74A) were cultured in 24-well plates and EC-VC or EC-Bcl-2 cells were plated on inserts and these inserts were carefully layered on top of 24-well plates containing tumor cells. After 72 h, tumor cell number was quantified. For neutralization study, anti-IL-8 antibody was added to 24-well plates at the time of coculture. *Represents a significant difference (P<0.05) as compared to the control group.
Tumors Populated with EC-Bcl-2 Show Enhanced Metastasis to Lungs
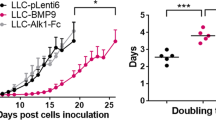
Our results from human cancer specimens suggest that Bcl-2 expression in tumor-associated ECs is directly linked to metastasis status in head-and-neck cancer patients. To investigate the role of Bcl-2 in tumor metastasis, we used the SCID mouse model and harvested lungs along with primary tumors at the end of the study period. The lungs' samples were immediately divided into two parts. One half of the lung was fixed and paraffin embedded for immunohistochemical analysis and the other half was treated with collagenase to harvest cells. Tumor cells (OSCC-3 and UM-SCC-74A) were selected with G418 treatment. After 7 days, the number of tumor cell colonies was counted. OSCC-3 tumors populated with EC-Bcl-2 cells showed significantly higher tumor metastasis to lungs as compared to OSCC-3 tumors populated with EC-VC cells (Figure 4a1–3). In addition to higher number of metastatic nodules, OSCC-3 tumors with EC-Bcl-2 also had larger lung metastatic tumor nodes. Although less metastatic than OSCC-3 tumors, UM-SCC-74A tumors populated with EC-Bcl-2 cells also showed marked increase in lung metastasis as compared to UM-SCC-74A tumors containing EC-VC cells (Figure 4b1–3). To further examine whether EC-Bcl-2-mediated tumor metastasis is independent of tumor growth, primary tumors from both EC-Bcl-2 and EC-VC groups were surgically removed once they reached 200 mm3 sizes and tumor metastasis to lungs was analyzed after 2 weeks. Indeed, EC-Bcl-2-mediated tumor metastasis was independent of primary tumor size (Figure 4c). In addition, in these animals, larger tumor lesions were observed in the lungs.
Tumor cells coimplanted with EC-Bcl-2 show marked increase in lung metastasis. (a and b) Lungs from SCID mice were carefully removed on day 21. One half of each lung was fixed and paraffin embedded for immunohistochemical analysis. Five paraffin-embedded step sections of 5 μm with 100 μm space were cut and stained with hematoxylin and eosin (H&E). The number of metastatic nodules present in these sections (a2: OSCC-3 and b2: UM-SCC-74A) was counted under microscope. From the other half of the lung, cells were harvested by collagenase (2.5 mg/ml) treatment. Tumor cells were selected by G418 treatment (200 μg/ml) for 1 week. The number of tumor cell colonies was counted (a3: OSCC-3 and b3: UM-SCC-74A). (c) Tumor and endothelial cells (ECs) were coimplanted in SCID mice as described in MATERIALS AND METHODS. Once the primary tumors reached 200 mm3, they were surgically removed. After 2 weeks, lungs from these animals were carefully removed and analyzed for metastasis as described above. (c1 and 3) Number of metastatic nodules present in OSCC-3 (c1) and UM-SCC-74A (c3) tumor groups. (c2 and 4) Number of tumor cell colonies present in OSCC-3 (c2) and UM-SCC-74A (c4) tumor groups. *Represents a significant difference (P<0.05) as compared to the control group.
Bcl-2 Expression in EC Leads to Increased Transendothelial Cell Permeability and Blood Vessel Leakiness
It is well established that tumor blood vessels are abnormally leaky. We next investigated whether Bcl-2-expressing ECs promoted blood vessel leakiness. We used both in vitro and in vivo models to examine the role of Bcl-2 in blood vessel leakiness. For in vitro studies, we used transendothelial cell permeability assay. ECs expressing Bcl-2 showed enhanced transendothelial cell permeability (Figure 5a). However, the difference observed with EC-Bcl-2 and EC-VC was not statistically significant. Tumor-associated ECs are normally exposed to a number of growth factors; it is therefore possible that the presence of these growth factors in the tumor microenvironment further enhances the blood vessel leakiness of Bcl-2-positive vessels. We next examined the effect of Bcl-2 on transendothelial cell permeability in the presence of VEGF. VEGF treatment of Bcl-2-expressing ECs significantly enhanced transendothelial cell permeability as compared to vector control cells (Figure 5b). To further corroborate the effect of Bcl-2 on blood vessel leakiness, we used normal angiogenesis as well as tumor angiogenesis models. Normal blood vessels that were populated with ECs expressing Bcl-2 showed marked increase in blood vessel leakiness as compared to blood vessels containing EC-VC cells (Figure 5c). Similarly, tumor blood vessels that were lined with ECs expressing Bcl-2 showed significantly higher blood vessel leakiness as compared to tumor blood vessels with EC-VC cells (Figure 5d).
Bcl-2 expression in endothelial cells (ECs) leads to increased transendothelial cell permeability as well as blood vessel leakiness in vivo. (a, b) EC-Bcl-2 or EC-VC cells (1 × 105) were cultured on top of 24-well transwell inserts coated with type 1 collagen to form a uniform layer. FITC-dextran (1 mg/ml, MW 40 000) solution was added to the transwell inserts in the presence (b) or absence (a) of VEGF. At different time points, 100 μl of supernatant was carefully removed from the bottom well and replaced with fresh media. The presence of FITC dextran in upper and lower wells was determined with a fluorometer, using an excitation wavelength of 492 nm, and detecting emission at 520 nm. (c) EC-Bcl-2 or EC-VC (2 × 106) were mixed with 500 μl of matrigel and implanted in the right and left flanks of SCID mice, respectively. After 10 days, 200 μl of FITC-dextran solution (25 mg/ml) was injected through the tail vein. After 30 min, matrigel plugs were carefully retrieved, fixed and frozen in OCT. Cryosections were analyzed by fluorescence microscope (c1–2) and the data are presented as vessel leakiness (c3). (d) OSCC-3 cells and EC-Bcl-2 or EC-VC (2 × 106) were mixed with 500 μl of matrigel and implanted in the right and left flanks of SCID mice, respectively. After 14 days, 200 μl of FITC-dextran solution (25 mg/ml) was injected through the tail vein. After 30 min, matrigel plugs were carefully retrieved, fixed and frozen in OCT. Cryosections were analyzed by fluorescence microscope (d1–2) and the data are presented as vessel leakiness (d3). *Represents a significant difference (P<0.05) as compared to the control group.
Bcl-2 Expression in EC Promotes Tumor Cell Invasion
We next examined whether Bcl-2 expression in ECs promotes tumor cell invasion. We used matrigel invasion assay. ECs expressing Bcl-2 or VC were plated on the bottom wells (24-well plates) and tumor cells (OSCC-3 or UM-SCC-74A) were cultured on top of matrigel-coated inserts. Bcl-2 expression in ECs induced marked increase in OSCC-3 invasion through the matrigel (Figure 6a–b). Similarly, UM-SCC-74A cells showed significantly higher invasion capacity when cultured with Bcl-2-expressing ECs (Figure 6c–d). We next examined whether EC-Bcl-2-mediated increase in tumor cell invasiveness is due to the production of IL-8. Neutralization of IL-8 by anti-IL-8 antibody significantly inhibited EC-Bcl-2-mediated tumor cell invasion (Figure 6).
Endothelial cells (ECs) expressing Bcl-2 promote tumor invasion. EC-Bcl-2 or EC-VC (50 000 cells/well) was cultured in 24-well plates. Next, 50 000 OSCC-3 (a, b) or UM-SCC-74A (c, d) cells were carefully layered on top of the matrigel-coated inserts and these inserts were then placed in the 24-well plates containing ECs. In neutralization study, anti-IL-8 antibody was added to bottom wells at the start of coculture. The plates were further incubated for 24 h at 37°C and the noninvaded cells were carefully removed with a cotton swab. The inserts were stained with Diff-quick solution II and the number of cells that had invaded through the matrigel was counted in five high power fields. *Represents a significant difference (P<0.05) as compared to the control group.
DISCUSSION
Tumor metastasis to vital organs still remains the major cause of death in cancer patients. Although our knowledge of the mechanisms of tumor progression has increased considerably in recent years, the role of tumor microenvironment in tumor metastasis is less well understood. By understanding the role of tumor accessory cells, particularly, ECs in tumor metastasis, it may be possible to design new strategies to block tumor metastasis to vital organs. We have previously shown that VEGF, a key angiogenic factor, can protect ECs against ionizing radiation by inducing the expression of Bcl-2.26 We have also shown that Bcl-2 expression in tumor-associated ECs promotes tumor progression.27 In our most recent work, we have demonstrated that Bcl-2, in addition to its well-documented role of regulating mitochondrial cytochrome c release, also mediates a signaling cascade to upregulate survivin expression through the Raf-MEK-ERK pathway.20 We also observed a significantly higher expression of Bcl-2 in tumor-associated blood vessels in head-and-neck cancer patients as compared to matched control samples.
In this study, we have further examined the role EC-Bcl-2 in tumor metastasis. Our results showed a significantly higher level of Bcl-2 expression in lymph node-positive patients. Out of all the different head-and-neck tumor types examined, tumor samples from patients with oral cavity tumors showed maximal Bcl-2 expression in tumor blood vessels followed by tumor samples from oropharynx, hypopharynx, glottic and supraglottic areas. This enhanced Bcl-2 expression pattern in head-and-neck tumor types could be due to the production of VEGF in these tumors as Bcl-2 expression profile in this study strongly correlated with VEGF profile as reported previously.31 Interestingly, even small tumors from stage II lymph node-positive patients exhibited significantly higher Bcl-2-positive blood vessels as compared to stage II lymph node negative patients. These results, therefore, suggest that Bcl-2 expression in tumor-associated ECs may have a role in tumor metastasis.
To more directly examine the role of Bcl-2 in tumor metastasis, we have employed both in vitro and in vivo models. Our results clearly demonstrate that Bcl-2 expression in tumor-associated ECs leads to significantly higher tumor growth and tumor metastasis. To examine whether EC-Bcl-2 promotes tumor growth in a paracrine manner, we performed coculture experiments. Our results indeed suggest that EC-Bcl-2 promoted tumor cell proliferation in a paracrine manner and this EC-Bcl-2-mediated tumor cell proliferation was partially but significantly mediated by IL-8. Similarly, Araki et al32 have shown that IL-8 induces significant prostate cancer cell (LNCap) proliferation through the CXCR1 receptor. We next examined whether higher tumor size in EC-Bcl-2-populated tumors were responsible for increased tumor metastasis in these animals. In this experiment, once the tumors reached 200 mm3 sizes, they were surgically removed and tumor metastasis in these animals was analyzed after 2 weeks. EC-Bcl-2-mediated tumor metastasis was found to be independent of primary tumor size. In addition, larger tumor lesions were found in the lungs. This increase in tumor lesion size in the lungs could be due to enhanced tumor angiogenesis resulted because of the decreased levels of angiogenesis inhibitors produced by primary tumors.33
Tumor cell invasiveness is a key characteristic of aggressive metastatic tumors as this plays an important role in tumor cell release from the primary tumors into the circulation. To examine whether ECs expressing Bcl-2 promote tumor cell invasion, we used a modified in vitro matrigel invasion assay. ECs transduced with either Bcl-2 or vector alone were cultured in the bottom wells and tumor cells were plated on top of the matrigel-coated inserts. Both tumor cell lines showed marked increase in tumor cell invasiveness, when cultured along with ECs expressing Bcl-2. We have previously shown that EC-Bcl-2 cells produce significantly higher amounts of IL-8 as compared to control cells.27 We next examined whether EC-Bcl-2-induced tumor cell invasion is mediated by IL-8. Neutralization of IL-8 by anti-IL-8 antibody significantly inhibited EC-Bcl-2-mediated tumor cell invasion. This could be due to IL-8-mediated tumor cell motility and increased production of matrix metalloproteinase-9 (MMP-9).32 These results suggest that soluble factors secreted by EC-Bcl-2 cells34 may promote tumor cell invasion and migration.35
Tumor blood vessels are usually quite different in their morphology and structure as compared to normal mature vessels.36 Tumor blood vessels often develop an irregular branching pattern,14 lack a proper basement membrane37 and are more leaky.16, 17 We investigated whether Bcl-2 expression in ECs promoted increased blood vessel leakiness. Indeed, ECs expressing Bcl-2 showed enhanced transendothelial cell permeability and blood vessel leakiness in vivo. Bcl-2-mediated transendothelial cell permeability and blood vessel leakiness could be due to its ability to directly bind to tubulin and modulate microtubule assembly38 or modulate adhesion molecule(s) expression by binding to BAG-1 protein and mediating a signaling cascade.20 Bcl-2 expression in tumor-associated ECs showed even higher blood vessel leakiness. This could be due to the presence of a number of angiogenic factors, particularly, VEGF in tumor microenvironment as treatment of Bcl-2-expressing endothelial monolayers with VEGF further enhanced transendothelial permeability.
In summary, our results suggest that Bcl-2 expression in tumor-associated ECs promotes tumor metastasis and this EC-Bcl-2-mediated tumor metastasis is independent of primary tumor size. Furthermore, our results suggest that EC-Bcl-2-mediated tumor metastasis could be due to EC-Bcl-2-mediated increase in tumor angiogenesis, tumor cell invasiveness and blood vessel leakiness. These results define an important new role for Bcl-2 protein and suggest that ECs expressing Bcl-2 may be a novel therapeutic target for tumor metastasis.
References
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000;100:57–70.
Bogenrieder T, Herlyn M . Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 2003;22:6524–6536.
Sporn MB . The war on cancer: a review. Ann NY Acad Sci 1997;833:137–146.
Folkman J . Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15–18.
Kirsch M, Schackert G, Black PM . Metastasis and angiogenesis. Cancer Treat Res 2004;117:285–304.
McDonnell CO, Hill AD, McNamara DA, et al. Tumour micrometastases: the influence of angiogenesis. Eur J Surg Oncol 2000;26:105–115.
Gasparini G, Weidner N, Maluta S, et al. Intratumoral microvessel density and p53 protein: correlation with metastasis in head-and-neck squamous-cell carcinoma. Int J Cancer 1993;55:739–744.
Toi M, Inada K, Suzuki H, et al. Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res Treat 1995;36:193–204.
Brawer MK . Quantitative microvessel density. A staging and prognostic marker for human prostatic carcinoma. Cancer 1996;78:345–349.
Angeletti CA, Lucchi M, Fontanini G, et al. Prognostic significance of tumoral angiogenesis in completely resected late stage lung carcinoma (stage IIIA-N2). Impact of adjuvant therapies in a subset of patients at high risk of recurrence. Cancer 1996;78:409–415.
Maeda K, Chung YS, Takatsuka S, et al. Tumor angiogenesis as a predictor of recurrence in gastric carcinoma. J Clin Oncol 1995;13:477–481.
Wiggins DL, Granai CO, Steinhoff MM, et al. Tumor angiogenesis as a prognostic factor in cervical carcinoma. Gynecol Oncol 1995;56:353–356.
Dvorak HF, Detmar M, Claffey KP, et al. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol 1995;107:233–235.
McDonald DM, Foss AJ . Endothelial cells of tumor vessels: abnormal but not absent. Cancer Metastasis Rev 2000;19:109–120.
Ito Y, Oike Y, Yasunaga K, et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res 2003;63:6651–6657.
Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000;156:1363–1380.
Baish JW, Netti PA, Jain RK . Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res 1997;53:128–141.
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002;2:647–656.
Antonsson B . Bax and other pro-apoptotic Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion. Cell Tissue Res 2001;306:347–361.
Kumar P, Coltas IK, Kumar B, et al. Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res 2007;67:1193–1202.
Wang HG, Takayama S, Rapp UR, et al. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci USA 1996;93:7063–7068.
Takayama S, Sato T, Krajewski S, et al. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell 1995;80:279–284.
Downward J . Ras signalling and apoptosis. Curr Opin Genet Dev 1998;8:49–54.
Garrington TP, Johnson GL . Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 1999;11:211–218.
Wiesenauer CA, Yip-Schneider MT, Wang Y, et al. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg 2004;198:410–421.
Kumar P, Miller AI, Polverini PJ . p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem 2004;279:43352–43360.
Nor JE, Christensen J, Liu J, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res 2001;61:2183–2188.
Kumar P, Benedict R, Urzua F, et al. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab Invest 2005;85:756–767.
Wojciak-Stothard B, Potempa S, Eichholtz T, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001;114:1343–1355.
Enis DR, Shepherd BR, Wang Y, et al. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci USA 2005;102:425–430.
Tae K, El-Naggar AK, Yoo E, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer Res 2000;6:2821–2828.
Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res 2007;67:6854–6862.
O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994;79:315–328.
Karl E, Warner K, Zeitlin B, et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-kappaB and CXC chemokines. Cancer Res 2005;65:5063–5069.
Ali S, Lazennec G . Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev 2007;26:401–420.
Jain RK . Molecular regulation of vessel maturation. Nat Med 2003;9:685–693.
Colorado PC, Torre A, Kamphaus G, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 2000;60:2520–2526.
Knipling L, Wolff J . Direct interaction of Bcl-2 proteins with tubulin. Biochem Biophys Res Commun 2006;341:433–439.
Acknowledgements
We thank Dr Douglas B Chepeha for providing tissue microarrays (TMA), Dr Thomas Carey for UM-SCC-74A cell line, Bhavna Kumar for technical assistance, and Biological Resources Branch, National Cancer Institute, NIH, for the rhVEGF. Grant support: University of Michigan's Head-and-Neck Cancer Specialized Program of Research Excellence (SPORE) Grant 5 P50 CA097248 (PK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P., Ning, Y. & Polverini, P. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest 88, 740–749 (2008). https://doi.org/10.1038/labinvest.2008.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2008.46
Keywords
This article is cited by
-
Combining radiation to EGFR and Bcl-2 blockade: a new approach to target cancer stem cells in head and neck squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2021)
-
A muscle-specific protein ‘myoferlin’ modulates IL-6/STAT3 signaling by chaperoning activated STAT3 to nucleus
Oncogene (2017)
-
RETRACTED ARTICLE: Effect of miR-451 on the Biological Behavior of the Esophageal Carcinoma Cell Line EC9706
Digestive Diseases and Sciences (2013)
-
BCL2 expression in CD105 positive neoangiogenic cells and tumor progression in angioimmunoblastic T-cell lymphoma
Modern Pathology (2012)
-
Inhibition of hemangioma development in a syngeneic mouse model correlates with bcl-2 suppression and the inhibition of Akt kinase activity
Angiogenesis (2012)