Abstract
Background:
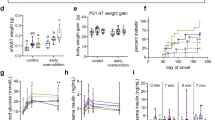
The intake of leptin during the suckling period protects against obesity and improves insulin and central leptin sensitivity in adult rats.
Objective:
We analyzed whether leptin treatment to neonates may also improve later peripheral leptin sensitivity in adipose tissue under high-fat (HF) diet conditions.
Design:
Male rats were supplemented with a daily oral dose of leptin or the vehicle (controls) during the suckling period. After weaning, animals were fed a normal-fat or an HF diet until the age of 6 months. At this age, mRNA and protein levels of the long-form leptin receptor (OB-Rb) and the expression of other genes related with energy metabolism were measured in various adipose depots (inguinal, mesenteric and retroperitoneal).
Results:
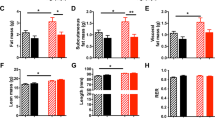
HF-diet feeding resulted in lower OB-Rb mRNA and protein levels in internal depots in controls but not in leptin-treated animals; these animals maintained OB-Rb mRNA and protein levels under HF-diet conditions in these depots, particularly in the mesenteric one, and this was accompanied by increased expression of genes related with energy uptake (GLUT4, CD36), fatty acid oxidation (peroxisome proliferator activated receptor-α (PPARα), CPT1, UCP3) and lipogenesis (PPARγ, GPAT). Leptin-treatment also ameliorated HF-diet-induced hepatic fat accumulation occurring in control animals.
Conclusion:
Leptin treatment during the suckling period may improve the lasting effects of HF-diet feeding on leptin receptor abundance in the adipose tissue and increase its oxidative capacity, resulting in a better handling and partitioning of excess fuel. This, together with the described improvement of central leptin sensitivity, may explain why these animals are more protected against diet-induced obesity and its metabolic-related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ahima RS, Flier JS . Leptin. Annu Rev Physiol 2000; 62: 413–437.
Ostlund Jr RE, Yang JW, Klein S, Gingerich R . Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 1996; 81: 3909–3913.
Margetic S, Gazzola C, Pegg GG, Hill RA . Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 2002; 26: 1407–1433.
Tartaglia LA . The leptin receptor. J Biol Chem 1997; 272: 6093–6096.
Fruhbeck G . Intracellular signalling pathways activated by leptin. Biochem J 2006; 393: 7–20.
Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC et al. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes 1998; 47: 538–543.
Sweeney G . Leptin signalling. Cell Signal 2002; 14: 655–663.
Ceddia RB . Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (London) 2005; 29: 1175–1183.
Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci USA 2004; 101: 2058–2063.
Zhang W, Della-Fera MA, Hartzell DL, Hausman D, Baile CA . Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life Sci 2008; 83: 35–42.
Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ . Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem 2003; 278: 45638–45650.
Wang MY, Orci L, Ravazzola M, Unger RH . Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA 2005; 102: 18011–18016.
Bouret SG, Draper SJ, Simerly RB . Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004; 304: 108–110.
Udagawa J, Hashimoto R, Hioki K, Otani H . The role of leptin in the development of the cortical neuron in mouse embryos. Brain Res 2006; 1120: 74–82.
Simerly RB . Hypothalamic substrates of metabolic imprinting. Physiol Behav 2008; 94: 79–89.
Palou A, Pico C . Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite 2009; 52: 249–252.
Miralles O, Sanchez J, Palou A, Pico C . A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006; 14: 1371–1377.
Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T et al. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes (London) 2007; 31: 1199–1209.
Oliver P, Pico C, De Matteis R, Cinti S, Palou A . Perinatal expression of leptin in rat stomach. Dev Dyn 2002; 223: 148–154.
Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A . Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005; 146: 2575–2582.
Sanchez J, Priego T, Palou M, Tobaruela A, Palou A, Pico C . Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 2008; 149: 733–740.
Weiss R . Fat distribution and storage: how much, where, and how? Eur J Endocrinol 2007; 157 (Suppl 1): S39–S45.
Palou M, Sanchez J, Priego T, Rodriguez AM, Pico C, Palou A . Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. J Nutr Biochem 2010; 21: 23–33.
Folch J, Lees M, Sloane Stanley GH . A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497–509.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Laemmli UK . Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685.
Fuster A, Pico C, Sanchez J, Oliver P, Zingaretti MC, Murano I et al. Effects of 6-month daily supplementation with oral beta-carotene in combination or not with benzo[a]pyrene on cell-cycle markers in the lung of ferrets. J Nutr Biochem 2008; 19: 295–304.
Priego T, Sanchez J, Pico C, Palou A . Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity (Silver Spring) 2008; 16: 819–826.
Fruhbeck G, Aguado M, Martinez JA . In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun 1997; 240: 590–594.
Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest 1997; 100: 2858–2864.
Seron K, Corset L, Vasseur F, Boutin P, Gomez-Ambrosi J, Salvador J et al. Distinct impaired regulation of SOCS3 and long and short isoforms of the leptin receptor in visceral and subcutaneous fat of lean and obese women. Biochem Biophys Res Commun 2006; 348: 1232–1238.
Priego T, Sanchez J, Palou A, Pico C . Effect of high-fat diet feeding on leptin receptor expression in white adipose tissue in rats: depot- and sex-related differential response. Genes Nutr 2009; 4: 151–156 .
Ceddia RB, William Jr WN, Lima FB, Flandin P, Curi R, Giacobino JP . Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem 2000; 267: 5952–5958.
Wang MY, Lee Y, Unger RH . Novel form of lipolysis induced by leptin. J Biol Chem 1999; 274: 17541–17544.
Palou M, Priego T, Sánchez J, Rodriguez AM, Palou A, Pico C . Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cell Physiol Biochem 2009; 24: 547–556.
Dulloo AG, Seydoux J, Jacquet J . Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav 2004; 83: 587–602.
Despres JP . Health consequences of visceral obesity. Ann Med 2001; 33: 534–541.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294: 2166–2170.
Palou A, Priego T, Pico C . Sex-dependent differences in lipid handling and the implications for obesity-linked disorders. Future Lipidology 2008; 3: 359–361.
Vidal-Puig AJ, Considine RV, Jimenez-Linan M, Werman A, Pories WJ, Caro JF et al. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 1997; 99: 2416–2422.
Rosen ED, Spiegelman BM . PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001; 276: 37731–37734.
Slawik M, Vidal-Puig AJ . Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 2006; 5: 144–164.
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002; 87: 3023–3028.
Dobrzyn A, Ntambi JM . The role of stearoyl-CoA desaturase in body weight regulation. Trends Cardiovasc Med 2004; 14: 77–81.
Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002; 297: 240–243.
Berti L, Kellerer M, Capp E, Haring HU . Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia 1997; 40: 606–609.
Ceddia RB, William Jr WN, Curi R . Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord 1999; 23: 75–82.
Dulloo AG, Stock MJ, Solinas G, Boss O, Montani JP, Seydoux J . Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett 2002; 515: 109–113.
Kus V, Prazak T, Brauner P, Hensler M, Kuda O, Flachs P et al. Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am J Physiol Endocrinol Metab 2008; 295: E356–E367.
Acknowledgements
This work was supported by the Spanish Government (Grant AGL2006-04887/ALI). Our Laboratory is a member of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: no. FP6-506360). The CIBER de Fisiopatología de la obesidad y nutrición is an initiative of the ISCIII.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Priego, T., Sánchez, J., Palou, A. et al. Leptin intake during the suckling period improves the metabolic response of adipose tissue to a high-fat diet. Int J Obes 34, 809–819 (2010). https://doi.org/10.1038/ijo.2010.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.18
Keywords
This article is cited by
-
Epigenetic programming for obesity and noncommunicable disease: From womb to tomb
Reviews in Endocrine and Metabolic Disorders (2023)
-
Leptin as a key regulator of the adipose organ
Reviews in Endocrine and Metabolic Disorders (2022)
-
Blood cell transcript levels in 5-year-old children as potential markers of breastfeeding effects in those small for gestational age at birth
Journal of Translational Medicine (2019)
-
Oral leptin supplementation throughout lactation in rats prevents later metabolic alterations caused by gestational calorie restriction
International Journal of Obesity (2017)
-
Blood cell transcriptomic-based early biomarkers of adverse programming effects of gestational calorie restriction and their reversibility by leptin supplementation
Scientific Reports (2015)