Abstract
Objective:
Relapsing to overeating is a stubborn problem in obesity treatment. We tested the hypothesis that context cues surrounding palatable food (PF) intake have the power to disrupt caloric regulation even of less PF. Context cues are non-food cues that are in the environment where PF is habitually eaten.
Design:
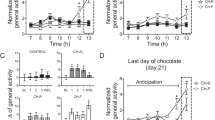
Rats were conditioned to associate intake of Oreo cookies as the PF to cages with distinct context cues that differed from cues in cages where they were only given chow. PF naturally stimulated greater caloric intake. The rats were then tested in the PF cage with only chow available to determine whether the PF-paired cues, alone, could elicit overeating of plain chow.
Subjects:
Non-food-deprived female Sprague–Dawley rats.
Measurements:
Intake of plain chow under PF-paired cues vs chow-paired cues was compared. This was also measured in tests that included a morsel of PF as a priming stimulus. We also controlled for any effect of binge-prone vs binge-resistant status to predict cued-overeating.
Results:
Rats consumed significantly more chow when exposed to context cues paired earlier with PF than with chow (P<0.01). This effect occurred using various cues (for example, different types of bedding or wallpaper). The effect was strengthened by priming with a morsel of PF (P<0.001) and was unaffected by baseline differences in propensity to binge on PF.
Conclusion:
Context-cues associated with PF intake can drive overeating even of a less PF and abolish the ability of rats to compensate for the calories of a PF primer. Just as drug-associated context cues can reinstate drug-addiction relapse, PF-paired cues may trigger overeating relapses linked to weight regain and obesity. This model should help identify the reflex-like biology that sabotages attempts to adhere to healthy reduced calorie regimens and call greater attention to the cue-factor in the treatment of binge eating and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr 2007; 85: 346–354.
Wadden TA . Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med 1993; 119: 688–693.
Hill JO, Wyatt HR, Reed GW, Peters JC . Obesity and the environment: where do we go from here? Science 2003; 299: 853–855.
Schlundt DG, Hill JO, Pope-Cordle J, Arnold D, Virts KL, Katahn M . Randomized evaluation of a low fat ad libitum carbohydrate diet for weight reduction. Int J Obes Relat Metab Disord 1993; 17: 623–629.
de Castro JM, Brewer EM . The amount eaten in meals by humans is a power function of the number of people present. Physiol Behav 1992; 51: 121–151.
Hetherington MM . Cues to overeat: psychological factors influencing overconsumption. Proc Nutr Soc 2007; 66: 113–123.
Raynor DA, Phelan S, Hill JO, Wing RR . Television viewing and long-term weight maintenance: results from the National Weight Control Registry. Obesity 2006; 14: 1816–1824.
Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R . Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite 2008; 51: 556–562.
Palfai TP, Macdonald A . Effects of temptations on the affective salience of weight control goals. Behav Res Ther 2007; 45: 449–458.
Wardle J . Conditioning processes and cue exposure in the modification of excessive eating. Addict Behav 1990; 15: 387–393.
Adam TC, Epel ES . Stress, eating, and the reward system. Physiol Behav 2007; 91: 441–458.
Thomas SL, Hyde J, Karunaratne A, Kausman R, Komesaroff PA . ‘They all work…when you stick to them’: a qualitative investigation of dieting, weight loss, and physical exercise in obese individuals. Nutr J 2008; 7: 34.
Lowe MG . The role of anticipated deprivation in overeating. Addict Behav 1982; 7: 103–112.
McGuire MT, Wing RR, Klem ML, Lang W, Hill JO . What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol 1999; 67: 177–185.
Niemeier HM, Phelan S, Fava JL, Wing RR . Internal disinhibition predicts weight regain following weight loss and weight loss maintenance. Obesity 2007; 15: 2485–2494.
Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR . Weight-loss maintenance in successful weight losers: surgical vs non-surgical methods. Int J Obes 2008; 33: 173–180.
Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA . Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr 2004; 79: 372–378.
Nasser J . Taste, food intake and obesity. Obes Rev 2001; 2: 213–218.
Hagan MM, Moss DE . Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord 1997; 22: 411–420.
Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K . A new animal model of binge-eating: key synergistic role of past caloric restriction and stress. Physiol Behav 2002; 77: 45–54.
Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK . Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci 2005; 119: 1207–1214.
Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD . The role of palatable food and hunger as trigger factors in an animal model of stress induced binge-eating. Int J Eat Disord 2003; 34: 183–197.
Levin BE, Dunn-Meynell AA . Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol 2002; 282: R46–R54.
Boggiano MM, Artiga AI, Pritchett CE, Chandler PC, Smith ML, Eldridge AJ . High intake of palatable food predicts binge-eating characteristics independent of susceptibility to obesity: an animal model of lean vs obese binge eating and obesity with and without binge-eating. Int J Obes 2007; 31: 1357–1367.
Siegel S, Hinson RE, Krank MD, McCully J . Heroin ‘overdose’ death: contribution of drug-associated environmental cues. Science 1982; 216: 436–437.
Siegel S, Ramos BM . Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharmacol 2002; 10: 162–183.
Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci 2001; 937: 1–26.
Taylor JR, Olausson P, Quinn JJ, Torregrossa MM . Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 2008; 56: 186–195.
Boggiano MM, Cavigelli SA, Dorsey JR, Pritchett-Kelley CE, Ragan CM, Chandler-Laney PC . Effect of a cage divider permitting social stimuli on stress and food intake in rats. Physiol Behav 2008; 95: 222–228.
Jarosz PA, Kessler JT, Sekhon P, Coscina DV . Conditioned place preferences (CPPs) to high-caloric ‘snack foods’ in rat strains genetically prone vs resistant to diet-induced obesity: resistance to naltrexone blockade. Pharmacol Biochem Behav 2007; 86: 699–704.
Weingarten HP . Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 1983; 220: 431–433.
Petrovich GD, Ross CA, Gallagher M, Holland PC . Learned contextual cue potentiates eating in rats. Physiol Behav 2007; 90: 362–367.
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL . Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci 2006; 26: 8531–8536.
Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y . The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharm 2006; 31: 2188–2196.
Hood S, Sorge RE, Stewart J . Chronic buprenorphine reduces the response to sucrose-associated cues in non food-deprived rats. Pharmacol Biochem Behav 2007; 86: 566–575.
Sobik L, Hutchison K, Craighead L . Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite 2005; 44: 253–261.
Lowe MR, Levine AS . Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res 2005; 13: 797–806.
Woods SC . Conditioned hypoglycemia. J Comp Physiol Psychol 1976; 90: 1164–1168.
Davidson TL, Swithers SE . A Pavlovian approach to the problem of obesity. Int J Obes Relat Metab Disord 2004; 28: 933–935.
Ferriday D, Brunstrom JM . How does food-cue exposure lead to larger meal sizes? Br J Nutr 2008; 100: 1325–1332.
Birch LL, McPhee L, Sullivan S, Johnson S . Conditioned meal initiation in young children. Appetite 1989; 13: 105–113.
Schachter S . Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science 1968; 161: 751–756.
Werrij MQ, Roefs A, Janssen I, Stapert D, Wolters G, Mulkens S et al. Early associations with palatable foods in overweight and obesity are not disinhibition related but restraint related. J Behav Ther Exp Psychiatry 2008; 40: 136–146.
Milton AL, Lee JL, Everitt BJ . Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem 2008; 15: 88–92.
Petrovich GD, Gallagher M . Control of food consumption by learned cues: a forebrain-hypothalamic network. Physiol Behav 2007; 24: 397–403.
Cohen DA . Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes 2008; 57: 1768–1773.
Vartanian LR, Herman CP, Wansink B . Are we aware of the external factors that influence our food intake? Health Psychol 2008; 27: 533–538.
Acknowledgements
We thank Dr Paul D Blanton for his editorial assistance. This work was funded in part by NIH Grant DK066007 and UAB Support for Development and Application of Research Using Animal Models (SDARAM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boggiano, M., Dorsey, J., Thomas, J. et al. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes 33, 693–701 (2009). https://doi.org/10.1038/ijo.2009.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.57
Keywords
This article is cited by
-
Food cue reactivity: Neurobiological and behavioral underpinnings
Reviews in Endocrine and Metabolic Disorders (2022)
-
Learned Overeating: Applying Principles of Pavlovian Conditioning to Explain and Treat Overeating
Current Addiction Reports (2018)
-
Eetverslaving
Bijblijven (2012)
-
Opiates, overeating and obesity: a psychogenetic analysis
International Journal of Obesity (2011)
-
Memantine reduces consumption of highly palatable food in a rat model of binge eating
Amino Acids (2011)