Abstract
Eighteen patients with refractory and progressive solid tumors were treated with a single round of triple modified oncolytic adenovirus (Ad5/3-Cox2L-D24). Ad5/3-Cox2L-D24 is the first non-Coxsackie-adenovirus receptor-binding oncolytic adenovirus used in humans. Grades 1–2 flu-like symptoms, fever, and fatigue were seen in most patients, whereas transaminitis or thrombocytopenia were seen in some. Non-hematological grades 3–5 side effects were seen in one patient with grade 3 ileus. Treatment resulted in high neutralizing antibody titers within 3 weeks. Virus appeared in serum 2–4 days after treatment in 83% of patients and persisted for up to 5 weeks. One out of five radiologically evaluable patients had partial response (PR), one had minor response (MR), and three had progressive disease (PD). Two patients scored as PD had a decrease in tumor density. Tumor reductions not measurable with Response Evaluation Criteria In Solid Tumors (RECIST) were seen in a further four patients. PR, MR, stable disease, and PD were seen in 12, 23.5, 35, and 29.5% of tumor markers analyzed, respectively (N=17). Ad5/3-Cox2L-D24 appears safe for treatment of cancer in humans and extended virus circulation results from a single treatment. Objective evidence of anti-tumor activity was seen in 11/18 (61%) of patients. Clinical trials are needed to extend these findings.
Similar content being viewed by others
Introduction
Oncolytic viruses take advantage of cancer-specific changes for preferential replication in tumor cells and are promising developmental agents for treating cancer refractory or incurable with available treatment modalities.1, 2, 3, 4 Replication causes oncolytic death of the cell, release of virions, and subsequent infection of surrounding cells, resulting in efficient tumor penetration and amplification of effect. Therefore, the anti-tumor effect is caused by replication per se. Inflammation caused by oncolysis may also have a key role in anti-tumor efficacy.5, 6, 7
Consequently, limiting replication to tumor cells may be important for reducing side effects. The anti-tumor effect of oncolytic viruses is determined in part by their capability for infection of tumor cells. Unfortunately, mounting evidence suggests that the expression level of the Coxsackie-adenovirus receptor is highly variable and often low in many human tumors.8 As most epidermal normal tissues express Coxsackie-adenovirus receptor, use of serotype 5 adenovirus may result in a suboptimal transduction profile. Nevertheless, even first generation oncolytic adenoviruses have shown some clinical utility.9, 10, 11 This suggests that if the capacity of the agents to transduce cancer cells could be enhanced, efficacy might be improved. Therefore, infectivity enhancement strategies have been studied.12
Two main approaches have been used to render the replication of oncolytic adenoviruses selective for tumor cells.13 ‘Transcomplementation regulated viruses’ such as dl1520 and Ad5-D24-RGD harbor partial early gene deletions transcomplemented in tumor but not normal cells. ‘Promoter controlled viruses’ feature tumor-specific promoters for control of early genes, most often E1A. Neither approach alone renders virus replication completely specific to target cells and therefore the combination of both may yield specificity benefits without loss of activity.12 Therefore, we constructed Ad5/3-Cox2L-D24, a triple mutant oncolytic adenovirus with the cyclooxygenase 2 (COX-2) promoter controlling E1A, a 24 bp constant region 2 deletion in E1A (for p16/Rb pathway selectivity) and the serotype 3 knob for enhancing tumor transduction.12, 14 Here, we report treatment of 18 cancer patients with Ad5/3-Cox2L-D24.
Results
Cox-2 expression in tumors
Archival tumor blocks were analyzed for cyclooxygenase-2 (Cox-2) protein expression (Table 1). Tumor samples from 15 out of 18 patients were available, and all except one (C6) immunostained positively for Cox-2. Strong, moderate, and weak immunopositivity were scored for 7, 4, and 3 patients, respectively.
One patient (P27) had malignant ascites that allowed COX-2 promoter expression and Ad5/3 transduction analysis on ascites cells. COX-2 expression could be detected (Figure 1a). Ad5/3 allowed a 12-fold increase in gene delivery compared to Ad5 (Figure 1b). Rb-p16 pathway mutations were not screened because such defects are probably present in all tumors, but 100% sensitive detection methods are not available.12, 15, 16, 17
Ascites cells of patient P27 were harvested before treatment. (a) Activity of COX-2 promoter was demonstrated by infecting cells in vitro with non-replicating chimeric Ad5Cox2L-luc1 adenovirus coding for luciferase. (b) In transduction analysis in vitro, chimeric Ad5/3luc1 showed 12-fold increase in gene delivery compared to Ad5luc1.
Adverse events
The treatment was generally well tolerated (Table 2). All patients experienced mild to moderate adverse events (AEs) after treatment, including fatigue, fever, and flu-like syndrome. In addition, five patients had grade 3 AEs. Four were asymptomatic hematological findings (hyponatremia, hypokalemia, INR (international normalized ratio for blood clotting) increase, thrombocytopenia) whereas one patient had grade 3 ileus. Ovarian cancer patient O37 was treated with 3 × 10e11 viral particles (VP). Twenty-one days later she was temporarily hospitalized because of reduction in intestinal motility. However, it is not clear if the ileus was caused by the treatment, or by the tumor, as intestinal problems are seen in nearly all patients with terminal ovarian cancer.18 At 4 weeks post treatment, she had increasing CA12-5 suggesting progressive disease. Moreover, as she was negative for virus on day 3, it may be that the virus had little effect in her case and that the intestinal problems were caused by tumor progression. There were no grades 4–5 side effects.
The correlation between different route of administration and AEs was analyzed. Absolute viral doses (VP) and the following four categories were correlated: (1) no AE, (2) grade 1, (3) grade 2, and (4) grade 3. Intraperitoneal dose correlated with a decrease in hemoglobin (P=0.05), vomiting (P=0.05), and stomach pain (P=0.01). Furthermore, intravenous dose correlated with hypertension (P=0.05), although only grade 1 hypertension was observed in three patients. No other correlations were observed.
Effect of Ad5/3-D24-Cox2L on liver enzymes and INR
All 18 patients were carefully evaluated for changes in aspartate aminotransferase, alanine aminotransferase, bilirubin, alkaline phosphatase, and thrombocyte counts after Ad5/3-Cox2L-D24 treatment (Supplementary Figure S1). Four patients (C6, R10, P20, P27) developed grade 2 increase in aspartate aminotransferase starting after 4–24 days of Ad5/3-Cox2-D24 treatment whereas three patients (R10, N21, H22) showed grade 2 increases in alanine aminotransferase. No grades 3–5 elevations were seen. There seemed to be no correlation between dose and transaminase increase (P=0.48 for aspartate aminotransferase; P=0.53 for alanine aminotransferase). Patients with elevated baseline values were more likely to have subsequent further increases.
Baseline measurements for bilirubin were within normal range (5–25 mmol l−1) expect for one patient (C6) showing bilirubin 26.8 mmol l−1 on the morning of treatment (Supplementary Figure S1c). This patient was treated with 6 × 10e9 VP and he showed grade 2 elevation in bilirubin (from 26.8 to peak value of 49.7 mmol l−1 on day 1). No overall association between transaminitis and hyperbilirubinemia was observed. In addition to C6, two patients (S4, C13) showed short-term grade 1 elevations in bilirubin 7 days (28.9 mmol l−1) and 18 days (27 mmol l−1) after treatment.
One patient (H5) had elevated INR (2.04) before treatment whereas 17 patients showed normal pretreatment measurement (Table 3). Five patients (S4, G11, N21, O25, P27) had mild (grade 1) increase in INR after treatment and one patient (R10) had grade 3 elevation in INR (pretreatment 1.0, 2.45 on day 17).
Clinical chemistry
One patient (P20) presented with grade 3 thrombocytopenia after treatment (Supplementary Figure S1e) and the lowest measured value was 31 × 10e9 l−1 2 weeks after treatment. However, his pretreatment thrombocyte count was 79 × 10e9 l−1 (scored as grade 2) meaning that the actual decrease after treatment was moderate. At baseline, 5 out of 18 patients (28%) had lower than normal thrombocyte counts (this is common in cancer patients). Four out of five had further decrease on day 1 after treatment (mean decrease 21%). Moreover, 8 out of 13 patients (62%) with normal pretreatment thrombocytes showed a decrease on day 1 (mean decrease 17%).
Typically for patients with advanced cancer, 13 out of 18 had low pretreatment Hb and erythrocytes (Table 3); 13 out of 18 patients had a small decrease (mean decrease 8%) in Hb on day 1. In addition, three patients had a decrease in Hb between days 4 and 17 (mean decrease 11%). Only one patient did not show any decrease in Hb during the 30 days follow-up period. Pretreatment leucocytes were mostly within normal range and no major changes occurred following the virus treatment.
Four out of 18 patients had low pretreatment serum potassium. One patient (G11) experienced grade 3 hypokalemia after treatment (from 3.7 to 2.5 mmol l−1 on day 10). This was treated with potassium supplementation that ultimately may have caused the grade 2 hyperkalemia seen on day 21 (5.9 mmol l−1). No other gradable changes in potassium occurred. For sodium, seven patients had low pretreatment measurement and in two of those (S4, R10) sodium decreased further (grade 3) post treatment. In addition, six patients showed mild (grade 1) decrease in sodium 3–24 days after the virus treatment.
One patient (O16) treated at dose 1 × 10e11 VP developed mild elevation in creatinine (grade 1) 25 days after treatment (from 73 to 104 μmol l−1) but otherwise creatinine values were within normal range. Only 8 out of 17 patients (47%) had normal c-reactive protein (CRP) before treatment and baseline values varied from normal (<10 mg l−1) to 294 mg l−1, as typical in patients with advanced cancer. Although c-reactive protein increases were regularly seen after treatment, little could be deduced from this given the frequent elevations at baseline.
Virus presence in the circulation
The presence of Ad5/3-Cox2L-D24 in the serum was analyzed by quantitative polymerase chain reaction (Table 4). All 18 patients were tested and they were all negative for the virus at baseline (day 0). All patients were evaluable after treatment and 15 had virus present in the serum in at least one measurement after treatment. In 11/14 evaluable cases, more virus were detected on day 2 or later, in comparison to day 1. The frequency of serum samples positive post treatment seemed to increase with the injected viral dose. With low viral dose (from 2.6 to 9 × 10e9 VP) only 20% (3 out of 15 samples) of analyzed post-treatment serum samples were positive. For the intermediate (from 1 to 5.3 × 10e10 VP) and high (from 1 to 3 × 10e11 VP) viral doses 59% (13 out of 22 samples) and 64% (18 out of 28 samples) of post-treatment serum samples were positive, respectively. Only three patients did not show viral genome in the circulation in any time points after the treatment.
Cytokines in circulation
Induction of serum IL-6, IL-8, IL-10, and TNF-α was analyzed at several time points after Ad5/3-Cox2L-D24 treatment (Supplementary Figure S2). Pretreatment values for serum IL-6 were analyzed for 16 patients and measurements varied between 13 and 245 pg ml−1. On the morning after treatment, serum IL-6 values were between 0 and 277 pg ml−1. Seven out of 16 patients showed a minor increase in IL-6 on day 1 (mean absolute increase 36 pg ml−1) compared to pretreatment value, but no dramatic increases were observed. For IL-8, pretreatment measurements were between 20 and 139 pg ml−1 and the corresponding values on day 1 were between 3 and 147 pg ml−1. Six patients showed a minor increase in IL-8 between day 0 (before treatment) and day 1 with the mean absolute increase of 36 pg ml−1. Serum IL-10 measurements before and 1 day after the treatment varied from 0–66 pg ml−1 to 0–49 pg ml−1, respectively. On day 1, six patients had slightly higher IL-10 value compared to pretreatment measurement (mean absolute increase 17 pg ml−1). Serum TNF-α measurements before virus treatment varied between 7 and 96 pg ml−1 and corresponding values 1 day after the treatment from 0 to 88 pg ml−1. With regard to TNF-α values before and 1 day after the treatment, five patients showed a minor increase with the mean absolute change of 37 pg ml−1. At later time point, no major changes in cytokine levels occurred. In summary, treatment with Ad5/3-Cox2L-D24 did not seem to cause major changes in serum IL-6, IL-8, IL-10, and TNF-α.
Neutralizing antibodies
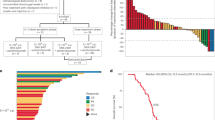
Serum samples of 17 patients could be analyzed for neutralizing antibodies (NAb) before and after treatment (Figure 2a). Before treatment, high anti-Ad5/3 antibody titers (1:16 384) were seen in 2/17 (12%), intermediate (1:4–1:256) in 12/17 (71%), and complete absence of detectable NAb was seen for 3/17 (18%) patients (H7, O16, and P27). Already by 2 weeks after treatment all patients had an increase in their NAb titers. NAb titers remained high until the end of follow-up (1–19 weeks) for all patients except for one (P20), who showed a minor decrease (from 1:16 384 to 1:4096) at 12 weeks post treatment.
(a) Neutralizing antibody (NAb) titer against chimeric adenovirus (Ad5/3) was measured from the serum of 17 patients before and after the virus administration. Data are presented as a serum dilution factor causing 80% inhibition in gene transfer with Ad5/3luc1 to 293 cells. (b) Survival plot of 18 Ad5/3-Cox2L-D24-treated patients. Median survival of patients was 106.5 days.
Anti-tumor responses
Five patients were evaluable for anti-tumor response by computed tomography (CT). An experienced radiologist compared the CT scans of patients taken before (within 3 weeks) and after (typically Circa 2 months) treatment. Modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria 18 were used to score the data. One patient with neuroblastoma (N21), treated with 1 × 10e11 VP, showed a partial response (71.1% reduction in tumor volume, 33.0% reduction in the longest diameter) in the treated primary tumor near the left kidney already 1 month after treatment (Figures 3a and b). Moreover, at 1 month his bone marrow aspirate was free of disease for the first time since diagnosis. Imaging was repeated at 3 months with identical results. This time, a more accurate bone marrow biopsy was performed instead of aspiration, and a small number of tumor cells could be detected.
(a) Computed tomography (CT) of primary tumor of patient N21 before Ad5/3-Cox2L-D24 treatment. (b) Magnetic resonance imaging of patient N21 1 month after treatment shows partial response of the primary tumor and complete regression of the lymph nodes. (c) CT of ovarian cancer patient O24 before treatment. (d) Minor response in patient O24 80 days after treatment. (e) Two scalp tumors (marked with arrows) of lung cancer patient K2 were injected with the virus. Picture was taken before treatment. (f) Complete response in the treated scalp tumors, confirmed later in autopsy, where no cancer cells were detected in the injected tumors.
Another patient (O24) had a minor response (41.4% reduction in the sum of tumor volumes, 12% reduction in sum of longest tumor diameters) after virus administration (Figures 3c and d). Three patients (C6, O9, C13) had more than 20% increase in the sum on tumor diameters in RECIST analysis and were therefore interpreted as progressive disease. However, two of these patients had a decrease in tumor density (C6: from 56.11 to 48.43; C13: from 52.28 to 47.75). This has been proposed to indicate anti-tumor effect even if tumor size does not decrease.19
Moreover, other evidence of anti-tumor activity was seen. In patient K2, we saw complete disappearance of injected scalp tumors and tumor hemorrhage also ceased concurrently (Figures 3e and f). Nevertheless, the patient eventually died from disease progression in the lungs and autopsy material became available. The area of treated scalp tumors was analyzed under the microscope and no viable tumor tissue was found. Nevertheless, PCR on the tissue at the injection site was positive for Ad5/3-Cox2L-D24. Moreover, the non-injected primary tumor in the lungs was found positive and trace amount of virus was also detected in the normal healthy liver.
In R10, the injected presternal tumor softened and in P27, an injected subcutaneous tumor decreased by 50% (clinical measurement). Patient S4 reported softening of the injected tumor. This, however, was not measurable and therefore is not counted as objective anti-tumor activity.
Fourteen patients were evaluated for tumor markers (total number of evaluable markers N=17) before and after treatment to assess the possible biological activity of Ad5/3-Cox2L-D24 (Figure 4). Eight patients showed a decrease in one or two of their tumor markers 1 week (or later) after virus administration. In addition, one patient (N21) showed a stabilization of tumor marker NSE for 12 weeks after the treatment. More than 30% reduction was seen in 12%, 12–29% reduction was seen in 23.5% whereas more than 20% increase was seen in 29.5% of markers; 35% of marker analyses did not fulfill the criteria for progression or response and were thus scored as stable disease. Therefore, tumor marker analysis suggested biological activity of the virus (defined as stability or reduction of the marker) in 70.5% of analyzable cases.
Overall, objective evidence (S4 not included) of anti-tumor efficacy was seen in 11/18 (61%) of patients (Table 4; Figures 3 and 4). These 11 did not have any difference in pre-existing NAb titer compared to patients with no objective benefit (P=0.65; t-test). One patient exhibited long-term survival and was alive when the manuscript was submitted, nearly 500 days after treatment (Figure 2b).
Discussion
More than 50 years ago, several wild-type viruses, including Ad3, were used in a pioneering clinical study,20 However, Ad5/3-Cox2L-D24 is the first non-Coxsackie-adenovirus receptor binding oncolytic (that is tumor selective) virus used in humans. It is also the first virus controlled both with a tumor-specific promoter and a transcomplementing mutation.12 High Cox-2 expression is a hallmark of many types of aggressive carcinomas.21 Here, Cox-2 expression was confirmed in archival tumor blocks in 94% of patients, and in one malignant ascites sample (Table 1; Figure 1).
To evaluate if Ad5/3-Cox2L-D24 would have utility in the context of personalized cancer treatment, we evaluated the correlation between archival tumor Cox-2 expression and serum Ad5/3-Cox2L-D24 genome copy number, but no correlation was seen (P=0.88). This may be because nearly all samples were positive for Cox-2. Moreover, it is possible that the treated tumors may have had a different Cox-2 expression profile from the archival specimens typically obtained from initial surgery years earlier. The only available fresh sample from patient P27 was positive for Cox-2 and highly transducable with Ad5/3. Interestingly, this patient showed a response in treated tumor (50% decrease in an injected tumor) and also considerable amount of viral DNA (up to 5 259 766 genomes per ml) was found in serum. Therefore, it might be worthwhile to study further if pretreatment biopsies might be useful for selecting patients for treatment.
We found that Ad5/3-Cox2L-D24 is relatively well tolerated up to the highest dose used in this study (3 × 10e11 VP). This dose is an order of magnitude lower than used previously with an oncolytic virus10 featuring a fully Ad5 capsid. Higher doses were not tested because the virus demonstrated anti-tumor activity even at the lowest doses used here and there are little data in the literature suggesting that a maximal tolerated dose would be needed for oncolytic viruses to be active. Further studies, however, may be needed to evaluate this further. Liver toxicity of adenovirus serotype 5 has been one of the main concerns in preclinical work,22 even if human cancer trials seem to suggest few liver problems.10, 11 Nevertheless, it is well established that human adenovirus does have the potential for human hepatotoxicity, when used in very high doses and when the liver is not functioning normally.23 In a Phase I trial with oncolytic adenovirus CG7870, five patients with metastatic prostate cancer were treated intravenously at a dose >1012 VP and all showed grade 1 or 2 transaminitis on days 2–8.10 Although the duration varied and some patients had elevated measurements as late as on day 22, peak values were typically measured between days 4 and 8.10 In our study, eight patients showed grade 1 or 2 transaminitis. Although values mostly recovered back to normal in 2 or 3 weeks, three patients showed elevated (grade 2) aspartate aminotransferase even after 3 or 4 weeks. As moderate baseline transaminase alterations were not the exclusion criteria, tumor-related enzyme elevation may have contributed to these findings. Importantly, and in accordance with earlier data from cancer patients, no severe (grade 3 or higher) transaminitis was observed and liver toxicity was not dose limiting in any way. An important contributing factor to this may have been the relatively low dose used here in contrast to other studies.10, 24
In line with earlier studies showing increased circulating IL-6, IL-8, and/or IL-10 levels in advanced cancer patients,25, 26 pretreatment values of these cytokines were somewhat elevated in the majority of patients. Rapid elevation in IL-6 or TNF-α indicates an acute inflammatory response and these cytokines may be sensitive markers of clinical or subclinical adenovirus toxicity.23, 27, 28, 29, 30 However, no significant increase in any of analyzed cytokines occurred in our patient cohort. This suggests that Ad5/3-Cox2L-D24, at the doses used here, did not induce acute systemic toxicity responses.23, 30
As proposed in previous investigations,31, 32, 33 we analyzed Ad5/3-Cox2L-D24 genome copy number in the serum as a surrogate for viral replication. We found virus in the serum of 13 out of 17 patients at least once 2 days or later post treatment, and in 11/14 evaluable cases, the amount increased as compared to day 1. Rapid clearance of adenovirus from the serum of humans has been shown in several studies,9, 10, 24 which led to the proposal that virus detected at 2 days or later may be due to virus replication.31, 32, 33 For example, DeWeese et al.9 showed that intraprostatically administered oncolytic adenovirus disappeared from the blood within 12 h. In that regard, our results showing positive serum samples up to day 38 post-treatment might reflect virus replication. However, collection of biopsies might shed more light on virus replication in tumors and might be useful in future studies.
In our study, the peak values for virus genomes were usually detected between days 2 and 10. This correlated well with previous results suggesting that most patients have their second peak of virus in blood 2 to 8 days post treatment (the first peak being associated with virus administration).9 However, two of our patients were positive starting on day 1 or 2 after virus administration and still had measurable viral loads even 37 or 38 days after the treatment, suggesting that in some patients virus replication and burst can begin rapidly, perhaps contributing to sustained virus titers in the serum. Only 3 out of 18 patients did not show virus in the circulation after the treatment. The dose might have affected virus measurements in these three patients as they were all treated with relatively low doses (from 6 × 10e9 to 2 × 10e10 VP) and our data suggest that a higher virus dose results in more prolonged virus presence in circulation. Moreover, others have showed prolonged viral detection in conjunction with higher dose.10
There is a strong body of clinical and preclinical evidence demonstrating an induction of NAb after adenovirus administration.9, 10, 24 Accordingly, we found a rapid induction of anti-Ad5/3 antibodies within 3 weeks of virus treatment and NAb titers remained high for several weeks or even months. Interestingly, only three patients were completely negative before treatment. Both serotype 3 and 5 adenoviruses are relatively common pathogens and some of the patients might have had wild-type 3 or 5 infections before Ad5/3-Cox2L-D24 treatment.
Preclinical data suggest that high levels of NAb might diminish the capacity of systemic (that is circulating) virus to transduce metastases, although NAb probably do not hinder intratumoral replication and oncolysis.34 In our study, patient N21, who had a 71.1% reduction in tumor size and complete clearance of bone marrow disease, had relatively low induction of NAb titer (1:1024) but the titer remained constant for 4.5 months after treatment. In contrast, O24 had a response 80 days after treatment but had rapid induction of a high NAb titer (1:16 384) and the titer remained high at least for 5 weeks. Overall, our results, together with most previous reports,9, 10, 24 suggest that despite high induction of NAbs, anti-tumor efficacy is achievable with oncolytic adenoviruses. In fact, it is well established that antibodies participate in immunological cell killing35 and there is emerging data that the anti-viral immune response may be an important part of the overall anti-tumor effect mediated by oncolytic viruses.5
In a study with oncolytic vaccinia virus, no correlation between baseline or post-treatment NAb titers and any clinical or laboratory end point including replication, GM-CSF expression, and efficacy, was observed.36 In our study, high NAb titer apparently did not prevent virus from replicating in tumors as both circulating Ad5/3-Cox2L-D24 genomes and increasing NAbs could be measured concurrently in many patients. In contrast to a previous report,9 we did not detect an inverse relationship between NAb titer at baseline and viral genomes in the serum 2–10 days later (P=0.72).
It seems likely that NAb induction is important from a safety perspective. Especially in patients with high tumor load, large cumulative amounts of infectious virus might shed into blood, with subsequent transduction of normal tissues, if it were not for the induction of NAb. As NAb induction was seen in all patients, the historical dogma of the immune suppressive state of late stage cancer patients may not apply in the context of anti-viral NAb responses. Nevertheless, it remains subject to further study if the anecdotal correlation between relatively low NAb induction and good efficacy in N21 was due to chance or hints at causative aspects.
With regard to efficacy, it may be promising that some evidence of biological activity of the virus could be detected in the majority of patients. Preclinical data suggest that modification of the adenovirus 5 capsid with the serotype 3 knob can enhance therapeutic potency in several tumor types.37, 38 Whether this had a role in the efficacy of Ad5/3-Cox2L-D24 cannot be evaluated without a randomized trial. Nevertheless, it is tantalizing that activity was seen with doses 10–100-fold lower than previously used with non-capsid modified oncolytic adenoviruses.9, 10, 39
One emerging understanding is that analyzing the efficacy of oncolytic viruses is challenging. Standard radiological criteria, developed for chemotherapy trials (for example, RECIST) may be suboptimal because virus replication causes local inflammation that enlarges tumors and could lead to incorrect interpretation of disease progression.24 Moreover, tumor markers may increase because of virus replication and tumor cells lysis.40 These findings, if confirmed, would mean that some of the patients interpreted as progressing may actually benefit from treatment. It has even been proposed that inflammation has a key role in the efficacy of oncolytic viruses.41, 42 These key aspects can only be fully understood in humans as all available animal models are defective either with regard to the immune environment (immune deficient and/or non-human), tumor niche (non-human or cell line xenograft often in a heterologous location), or permissivity to human adenovirus replication.
Positron emission tomography or magnetic resonance imaging are unlikely to solve these issues as both methods are sensitive to inflammation. Measurements of tumor density may provide some benefits,19, 36 but remain unproven for most tumor and treatment types. Importantly, as tumor and marker responses are just surrogate criteria, evaluating the most relevant end points (quality of life, overall survival) is not more difficult for oncolytic viruses in comparison to other therapeutics. However, these end points do require a randomized trial, which has so far only been achieved with one oncolytic virus.11
It may be promising that a single round of treatment led to persistent virus circulation in many patients, suggesting effective virus replication, despite rather conservative dosing. This finding raises the possibility that extremely high doses may not be required, if sufficiently potent viruses are used and if intratumoral injection is used to harness tumors as ‘virus factories’. In a previous study, high intravenous dose ultimately resulted in toxicity, which abrogated dose escalation.10 In contrast, preclinical data from mice suggest that even small doses can be effective because the virus replicates.37 Nevertheless, data from immune-deficient animals need to be interpreted with caution as the NAb response may allow only a few weeks of ‘window of opportunity’ for the virus to transduce distant tumors in immune competent systems.
Although preliminary data reported here suggest some anti-tumor activity for Ad5/3-Cox2L-D24, room for improvement remains. Better results may be possible in patients with less extensive disease. Moreover, repeat administration might be useful for improving tumor transduction. Nevertheless, our data suggest that capsid modified and double controlled oncolytic adenoviruses are a rational and safe platform for further improvements in efficacy. Such improvements can be realized by combining virus with standard treatments, which can lead to synergistic results, especially in non-refractory tumors.37, 43, 44, 45 Alternatively, or in addition, oncolytic viruses can be armed with therapeutic transgenes, in an approach with combines the benefits of both cancer gene therapy and oncolytic virotherapy.46, 47, 48 Further, immune suppression could yield benefits by slowing NAb induction or modulating the immunological tumor environment, although a concomitant increase in toxicity is also possible.49, 50
In summary, our data suggest that treatment of cancer patients with Ad5/3-Cox2L-D24 is safe and may be effective in some cases. Further studies are needed to evaluate both aspects.
Materials and methods
Patients
Eighteen patients were treated with a single round of virus at doses from 2 × 10e9 to 3 × 10e11 VP intratumorally or—in the case of intraperitoneal disease—intraperitoneally in ultrasound guidance. Depending on the location of the tumor, part of the dose was also given intravenously. Inclusion criteria were solid tumor (not leukemia or lymphoma), refractory and progressing disease previously treated with oncology treatments for which there is strong scientific evidence, written informed consent, and no major organ function deficiencies. Tumors likely to be Cox-2 positive and defective in the p16/Rb pathway were included.51, 52, 15 Other oncological treatments were not administered concurrently. Exclusion criteria were organ transplant, HIV, severe cardiovascular, metabolic, or pulmonary disease (for example, symptomatic coronary heart disease, uncontrolled blood pressure). The study was completed according to Good Clinical Practice and the Declaration of Helsinki. Cancer type, tumor Cox-2 status, diagnosis, sex, age, WHO status at the time of treatment, and prior treatments are summarized in Table 1. This compassionate use scheme was approved by the Medicolegal Department of the Finnish Ministry of Social Affairs and Health and The Gene Technology Board. Patients were monitored overnight at the hospital and as outpatients thereafter, for a minimum of 4 weeks. Side effects were recorded according to CTCEA 3.0. As many cancer patients have symptoms because of disease, pre-existing symptoms were not listed as side effects if they did not become worse. However, if the symptom became more severe, for example pretreatment grade 1 changed to grade 2 after treatment, it was scored as grade 2. Patients were imaged before and after treatment and modified RECIST criteria (when applicable) were used to evaluate anti-tumor efficacy.53 These criteria are partial response (>30% reduction in the sum of tumor diameters), minor response (12–29% reduction), stable disease (no response/progression), progressive disease (>20% increase). For tumor markers, the same percentages were used. Tumor density was measured as reported.19
Virus
Ad5/3-Cox2L-D2412, 14 is selective for Cox-2 expressing and Rb/p16 pathway mutant cells. A 24 bp deletion (‘D24’) in the constant region 2 of adenovirus E1A renders the virus unable to bind Rb and therefore allows virus replication only in Rb/p16 mutant tumor cells. Further selectivity is achieved by replacing the native E1A promoter with the COX-2 promoter. The biodistribution and toxicity of Ad5/3 chimeric adenovirus has been studied in mice and semipermissive Syrian hamsters,54 (Hemminki A, personal communication, 3 June 2009). Virus was produced on A549 cells to avoid the risk of recombination with transcomplementing sequences. The VP titer of Ad5/3-Cox2L-D24 was 2 × 10e12 VP per ml and the functional titer—as estimated by TCID50 method—was 1.3 × 10e11 p.f.u. per ml resulting in a genome to p.f.u. ratio (VP/p.f.u.) of 15.4. The conversion factor of 0.7 was used to transform TCID50 value to p.f.u. value as suggested in the AdEasy protocol (Quantum Biotechnology; Qbiogen, Carlsbad, CA, USA). Virus stock buffer formulation was 10 mM Trizmabase, 75 mM NaCl, 5% (w/v) sucrose, 1 mM MgCl, 10 mM L(+)histidine, 0.5% (v/v) EtOH, 0.02% Tween, 100 μM EDTA; 0.9% (w/v) NaCl solution (B Braun, Melsungen, Germany) was used as a diluent. Injection volume was 10 ml typically and injection sites were decided patient-by-patient with the aim of injecting the largest tumors. Intravenous bolus injection was performed in the superficial veins of the upper extremities, if a central venous catheter was not available.
Serum cytokine analysis
Cytokine analysis was performed with BD Cytometric Bead Array Human Soluble Protein Flex Set (Becton Dickinson, Franklin Lakes, NJ, USA) according to manufacturer's instructions. Patients’ serum samples were used for measurements.
Neutralizing antibody titer determination
Two hundred and ninety-three cells were seeded at 1 × 104 cells per well on 96-well plates and cultured overnight. Next day, cells were washed with DMEM without FCS. Human serum samples were incubated at 56 °C for 90 min to inactivate complement, and a fourfold dilution series (1:1–1:16 384) was prepared in serum-free DMEM.55 Ad5/3luc154 was mixed with serum dilutions and incubated at room temperature for 30 min. Thereafter, cells in triplicates were infected with 100 VP per cell in 50 μl of mix, and 100 μl of growth medium with 10% FCS was added 1 h later. 24 h post infection, cells were lysed and luciferase activity was measured with Luciferase Assay System (Promega, Madison, WI, USA) using TopCount luminometer (PerkinElmer, Waltham, MA, USA). Luciferase readings were plotted relative to gene transfer achieved with Ad5/3luc1 alone. The NAb titer was determined as the lowest degree of dilution that blocked gene transfer more than 80%.
Detection of viral DNA in serum samples
Total DNA was extracted by adding 3 μg of carrier DNA (polydeoxyadenylic acid; Roche, Mannheim, Germany) to 400 μl of serum and using the QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany). Extracted DNA was eluted in 60 μl nuclease-free water and DNA concentration was measured by spectrophotometry. PCR amplification was based on primers and probe targeting the E1A region flanking the 24 bp deletion (forward primer 5′-TCCGGTTTCTATGCCAAACCT-3′, reverse primer 5′-TCCTCCGGTGATAATGACAAGA-3′ and probe onco 5′FAM-TGATCGATCCACCCAGTGA-3′MGBNFQ). In addition, a probe complementary to a sequence included in the 24 bp region targeted for deletion was used to test the samples for the presence of wild-type adenovirus infection (probe wt 5′VIC-TACCTGCCACGAGGCT-3′MGBNFQ).
The real-time PCR conditions for each 25 μl reaction were as follows: 2X LightCycler480 Probes Master Mix (Roche, Mannheim, Germany), 800 nM each forward and reverse primer, 200 nM each probe, and 250 ng extracted DNA. PCR reactions were carried out in a LightCycler (Roche) under the following cycling conditions: 10 min at 95 °C, 50 cycles of 10 s at 95 °C, 30 s at 62 °C, and 20 s at 72 °C and 10 min at 40 °C. All samples were tested in duplicate. TaqMan exogenous internal positive control reagents (Applied Biosystems, Carlsbad, CA, USA) were used in the same PCR runs to test each sample for the presence of PCR inhibitors. A regression standard curve was generated using DNA extracted from serial dilutions of Ad5/3-D24-Cox2L (1 × 1E8–10 VP per ml) in normal human serum. The limit of detection and limit of quantification for the assay were 500 VP per ml of serum. All positive samples were further confirmed by real-time PCR using LightCycler480 SYBR Green I Master mix (Roche) and primers specific for COX-2L and adenovirus sequences, respectively (forward primer 5′-CACGTCCAGGAACTCCTCAG-3′ and reverse primer 5′-CGGCCATTTCTTCGGTAATA-3′).
Cox-2 staining of tumors
Formalin-fixed, paraffin-embedded archival tumor samples were obtained for 14 patients; 4 μm tissue sections were stained using monoclonal mouse anti-human Cox-2 (Cayman, Cat.no160112, Ann Arbor, MI, USA). Sections were deparaffinized, rehydrated, and heated in a microwave oven in 50 mM Tris–HCl buffer (pH 8.5) for 4 × 5 min for antigen retrieval. To block endogenous peroxidase activity, sections were treated with 0.3% H2O2 in methanol for 30 min. After blocking and washing with phosphate-buffered saline the sections were incubated overnight at +4 °C with the antibody (1:200) diluted in phosphate-buffered saline including 0.1%NaN3 and 0.5% bovine serum albumin. For secondary antibody labeling, the avidin–biotin method was used as described in the Vectastain ABC -kit (Vector Laboratories, Burlingame, CA, USA). Diaminobezidine was used as a chromogen. All sections were counterstained with hematoxylin (Gill NO.1 Sigma-Aldrich, St Louis, MO, USA) and mounted with DePeX (Electron Microscopy Sciences, Hatfield, PA, USA). The primary antibody was omitted in the negative control. Tumor tissue, in which Cox-2 expression was previously confirmed to be strong, was used as a positive control. Cox-2 staining was evaluated with light microscopy by a pathologist with no previous information about the patient's clinical status. Staining intensity of cancer cells was scored as follows: Negative=−, Weak=1+, Moderate=2+, Strong=3+.
Cox-2 promoter expression and Ad5/3 transduction analysis of ascites cells in vitro
Ascites cells were washed twice with DMEM and 1 × 10e5 cells per well were plated on 24-well plates. On the next day, cells were infected for 30 min at room temperature at 40, 200, 1000, or 5000 VP per cell by adding replication deficient, luciferase expressing virus Ad5cox2L-Luc1 diluted in 200 μl of DMEM with 2% FCS. Infection medium was replaced with DMEM containing 10% FCS and cells were incubated for 24 h at 37 °C. Cells were then lysed with reporter lysis buffer (Promega) and frozen at −80 °C. Luciferase activity was measured according to manufacturer's manual (Luciferase Assay System, Promega).
For Ad5/3 transduction analysis, 1 × 10e5 ascites cells per well on 24-well plates were infected with replication-deficient luciferase coding viruses Ad5luc1 or Ad5/3luc156 at viral dose of 1000 VP per cell and luciferase activity was assessed as described above.
Statistical analysis
All analyses were done with SPSS 15.0 software for Windows. Correlations between baseline neutralizing antibody titer and viral genomes in the serum and between viral dose and transaminase increase were analyzed with Pearson Correlation analysis. Non-parametric Spearman's Rho analysis was used to evaluate correlations between archival tumor Cox-2 expression and viral genomes in the serum, and between route of administration and AEs. A P-value <0.05 was considered the limit for statistical significance.
References
Alemany R . Cancer selective adenoviruses. Mol Aspects Med 2007; 28: 42–58.
Stone D, Lieber A . New serotypes of adenoviral vectors. Curr Opin Mol Ther 2006; 8: 423–431.
Lin E, Nemunaitis J . Oncolytic viral therapies. Cancer Gene Therapy 2004; 11: 643–664.
Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE . Oncolytic viruses in cancer therapy. Cancer Lett 2007; 254: 178–216.
Alemany R, Cascallo M . Oncolytic viruses from the perspective of the immune system. Future Microbiol 2009; 4: 527–536.
Di Paolo NC, Tuve S, Ni S, Hellström KE, Hellström I, Lieber A . Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res 2006; 66: 960–969.
Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 2009.
Rein DT, Breidenbach M, Curiel DT . Current developments in adenovirus-based cancer gene therapy. Future Oncol 2006; 2: 137–143.
DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res 2001; 61: 7464–7472.
Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther 2006; 14: 107–117.
Yu W, Fang H . Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets 2007; 7: 141–148.
Bauerschmitz GJ, Guse K, Kanerva A, Menzel A, Herrmann I, Desmond RA et al. Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol Ther 2006; 14: 164–174.
Alemany R, Balague C, Curiel DT . Replicative adenoviruses for cancer therapy. Nat Biotechnol 2000; 18: 723–727.
Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M et al. Tissue-specific promoters active in CD44+CD24-/low breast cancer cells. Cancer Res 2008; 68: 5533–5539.
Sherr CJ . Cancer cell cycles. Science 1996; 274: 1672–1677.
Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000; 19: 2–12.
Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med 2000; 6: 1134–1139.
Rousseau P . Management of malignant bowel obstruction in advanced cancer: a brief review. J Palliat Med 1998; 1: 65–72.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007; 25: 1753–1759.
Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB . Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 1956; 9: 1211–1218.
Wang D, Dubois RN . Prostaglandins and cancer. Gut 2006; 55: 115–122.
Kojaoghlanian T, Flomenberg P, Horwitz MS . The impact of adenovirus infection on the immunocompromised host. Rev Med Virol 2003; 13: 155–171.
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–158.
Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res 2002; 62: 6070–6079.
Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 2009; 18: 215–222.
Kotowicz B, Fuksiewicz M, Kowalska M, Jonska-Gmyrek J, Bidzinski M, Kaminska J . The value of tumor marker and cytokine analysis for the assessment of regional lymph node status in cervical cancer patients. Int J Gynecol Cancer 2008; 18: 1279–1284.
Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther 2007; 15: 378–385.
Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther 2009; 17: 327–333.
Brunetti-Pierri N, Palmer DJ, Mane V, Finegold M, Beaudet AL, Ng P . Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol Ther 2005; 12: 99–106.
Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P . Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Therapy 2004; 15: 35–46.
Nemunaitis J, Senzer N, Sarmiento S, Zhang Y, Arzaga R, Sands B et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Therapy 2007; 14: 885–893.
Nemunaitis J, Meyers T, Senzer N, Cunningham C, West H, Vallieres E et al. Phase I Trial of sequential administration of recombinant DNA and adenovirus expressing L523S protein in early stage non-small-cell lung cancer. Mol Ther 2006; 13: 1185–1191.
Galanis E, Okuno S, Nascimento A, Lewis B, Lee R, Oliveira A et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Therapy 2005; 12: 437–445.
Dhar D, Spencer JF, Toth K, Wold WS . Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol 2009; 83: 2130–2139.
Strome SE, Sausville EA, Mann D . A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist 2007; 12: 1084–1095.
Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 2008; 9: 533–542.
Raki M, Sarkioja M, Desmond RA, Chen DT, Butzow R, Hemminki A et al. Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer. Gynecol Oncol 2008; 108: 166–172.
Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther 2003; 8: 449–458.
Kirn D . Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Therapy 2001; 8: 89–98.
Turunen T NP, Cerullo V, Pesonen S, Oksanen M, Escutenaire S, Hemminki A . Effect of oncolytic adenovirus on tumor marker levels in cancer patients and in preclinical test systems. Mol Ther 2009; 17(Suppl 1): S110.
Alemany R . A smart move against cancer for vaccinia virus. Lancet Oncol 2008; 9: 507–508.
Tuve S, Liu Y, Tragoolpua K, Jacobs JD, Yumul RC, Li ZY et al. In situ adenovirus vaccination engages T effector cells against cancer. Vaccine 2009; 27: 4225–4239.
Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res 2001; 61: 5453–5460.
Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Therapy 2005; 12: 715–722.
Liu D, Kojima T, Ouchi M, Kuroda S, Watanabe Y, Hashimoto Y et al. Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol Cancer Ther 2009; 8: 980–987.
Kirn DH, Thorne SH . Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 2009; 9: 64–71.
Todo T . Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci 2008; 13: 2060–2064.
Hermiston TW, Kuhn I . Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Therapy 2002; 9: 1022–1035.
Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS . Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther 2008; 16: 1665–1673.
Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA 2008; 105: 7293–7297.
Juuti A, Louhimo J, Nordling S, Ristimaki A, Haglund C . Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol 2006; 59: 382–386.
D’Andrilli G, Kumar C, Scambia G, Giordano A . Cell cycle genes in ovarian cancer: steps toward earlier diagnosis and novel therapies. Clin Cancer Res 2004; 10: 8132–8141.
Warren KE, Patronas N, Aikin AA, Albert PS, Balis FM . Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst 2001; 93: 1401–1405.
Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 2002; 5: 695–704.
Sarkioja M, Pesonen S, Raki M, Hakkarainen T, Salo J, Ahonen MT et al. Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies. Gene Therapy 2008; 15: 921–929.
Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8: 275–280.
Acknowledgements
We thank Sirkka-Liisa Holm and Päivi Hannuksela (Cancer Gene Therapy Group, University of Helsinki, Finland), Saila Eksymä-Sillman, Marina Rosliakova, and Kylli Skogström (Eira Hospital, Helsinki, Finland), Satu Nikander, Arja Vilkko, Heini Välijeesiö, Jenni Kylä-Kause, Katri Silosuo, and other nurses at International Comprehensive Cancer Center Docrates, Helsinki, Finland, and at Eira Hospital. Akseli Hemminki is K Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Pesonen, S., Nokisalmi, P., Escutenaire, S. et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther 17, 892–904 (2010). https://doi.org/10.1038/gt.2010.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.17
Keywords
This article is cited by
-
Untangling the Intricacies of Infection, Thrombosis, Vaccination, and Antiphospholipid Antibodies for COVID-19
SN Comprehensive Clinical Medicine (2021)
-
Recombinant viruses with other anti-cancer therapeutics: a step towards advancement of oncolytic virotherapy
Cancer Gene Therapy (2018)
-
Predictive and Prognostic Clinical Variables in Cancer Patients Treated With Adenoviral Oncolytic Immunotherapy
Molecular Therapy (2016)
-
Attenuated Semliki Forest virus for cancer treatment in dogs: safety assessment in two laboratory Beagles
BMC Veterinary Research (2015)
-
Biodistribution Analysis of Oncolytic Adenoviruses in Patient Autopsy Samples Reveals Vascular Transduction of Noninjected Tumors and Tissues
Molecular Therapy (2015)