Abstract
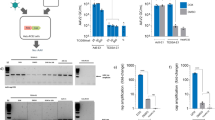
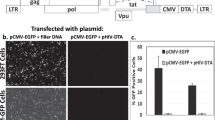
Large-scale production of gene therapeutics comprising equine infectious anaemia virus (EIAV) -based lentiviral vectors (LVs) would benefit from the development of producer cell lines enabling the generation of larger quantities of vector than achievable by transient systems. Such cell lines would contain three vector components (Gag/Pol, VSV-G envelope and genome expression constructs). As the vesicular stomatitis virus (VSV-G) envelope protein is cytotoxic, its expression must be regulated. It is also desirable to regulate Gag/Pol expression to minimise metabolic burden on the cell. The Tet repressor (TetR) system was selected to regulate expression of VSV-G and Gag/Pol, necessitating the introduction of a fourth construct, encoding TetR, into the cell line. We have generated an inducible packaging cell line that shows tight control of the packaging components, and high-titre vector production on transient transfection of the EIAV genome. The cell line is stable for at least 7 weeks in the absence of selective pressure. To verify that this packaging cell line can support the generation of producer cell lines it was transfected stably with an EIAV genome cassette encoding ProSavin; a gene therapeutic for Parkinson's disease. Producer cell lines were generated, which on induction, yielded ProSavin with titres comparable to the transient system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet 2001; 10: 2109–2121.
Mitrophanous K, Yoon S, Rohll J, Patil D, Wilkes F, Kim V et al. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Therapy 1999; 6: 1808–1818.
Mselli-Lakhal L, Guiguen F, Greenland T, Mornex JF, Chebloune Y . Gene transfer system derived from the caprine arthritis-encephalitis lentivirus. J Virol Methods 2006; 136: 177–184.
Poeschla EM, Wong-Staal F, Looney DJ . Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med 1998; 4: 354–357.
Takahashi K, Luo T, Saishin Y, Saishin Y, Sung J, Hackett S et al. Sustained transduction of ocular cells with a bovine immunodeficiency viral vector. Hum Gene Ther 2002; 13: 1305–1316.
Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB et al. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci 2002; 22: 10302–10312.
Azzouz M, Mazarakis N . Non-primate EIAV-based lentiviral vectors as gene delivery system for motor neuron diseases. Curr Gene Ther 2004; 4: 277–286.
Azzouz M, Ralph S, Wong LF, Day D, Askham Z, Barber RD et al. Neuroprotection in a rat Parkinson model by GDNF gene therapy using EIAV vector. Neuroreport 2004; 15: 985–990.
Balaggan KS, Binley K, Esapa M, MacLaren RE, Iqball S, Duran Y et al. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Therapy 2006; 13: 1153–1165.
Bienemann AS, Martin-Rendon E, Cosgrave AS, Glover CP, Wong LF, Kingsman SM et al. Long-term replacement of a mutated nonfunctional CNS gene: reversal of hypothalamic diabetes insipidus using an EIAV-based lentiviral vector expressing arginine vasopressin. Mol Ther 2003; 7: 588–596.
Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Therapy 2008; 15: 1311–1320.
Radcliffe PA, Sion CJ, Wilkes FJ, Custard EJ, Beard GL, Kingsman SM et al. Analysis of factor VIII mediated suppression of lentiviral vector titres. Gene Therapy 2008; 15: 289–297.
Ralph GS, Binley K, Wong LF, Azzouz M, Mazarakis ND . Gene therapy for neurodegenerative and ocular diseases using lentiviral vectors. Clin Sci (Lond) 2006; 110: 37–46.
Broussau S, Jabbour N, Lachapelle G, Durocher Y, Tom R, Transfiguracion J et al. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol Ther 2008; 16: 500–507.
Cockrell AS, Ma H, Fu K, McCown TJ, Kafri T . A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Mol Ther 2006; 14: 276–284.
Farson D, Witt R, McGuinness R, Dull T, Kelly M, Song J et al. A new-generation stable inducible packaging cell line for lentiviral vectors. Hum Gene Ther 2001; 12: 981–997.
Ikeda Y, Takeuchi Y, Martin F, Cosset FL, Mitrophanous K, Collins M . Continuous high-titer HIV-1 vector production. Nat Biotechnol 2003; 21: 569–572.
Kafri T, van Praag H, Ouyang L, Gage FH, Verma IM . A packaging cell line for lentivirus vectors. J Virol 1999; 73: 576–584.
Kaul M, Yu H, Ron Y, Dougherty JP . Regulated lentiviral packaging cell line devoid of most viral cis-acting sequences. Virology 1998; 249: 167–174.
Klages N, Zufferey R, Trono D . A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther 2000; 2: 170–176.
Ni Y, Sun S, Oparaocha I, Humeau L, Davis B, Cohen R et al. Generation of a packaging cell line for prolonged large-scale production of high-titer HIV-1-based lentiviral vector. J Gene Med 2005; 7: 818–834.
Pacchia AL, Adelson ME, Kaul M, Ron Y, Dougherty JP . An inducible packaging cell system for safe, efficient lentiviral vector production in the absence of HIV-1 accessory proteins. Virology 2001; 282: 77–86.
Sparacio S, Pfeiffer T, Schaal H, Bosch V . Generation of a flexible cell line with regulatable, high-level expression of HIV Gag/Pol particles capable of packaging HIV-derived vectors. Mol Ther 2001; 3: 602–612.
Xu K, Ma H, McCown TJ, Verma IM, Kafri T . Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol Ther 2001; 3: 97–104.
Yu H, Rabson AB, Kaul M, Ron Y, Dougherty JP . Inducible human immunodeficiency virus type 1 packaging cell lines. J Virol 1996; 70: 4530–4537.
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK . Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 1993; 90: 8033–8037.
Cronin J, Zhang XY, Reiser J . Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 2005; 5: 387–398.
DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther 2000; 2: 218–222.
Yee JK, Miyanohara A, LaPorte P, Bouic K, Burns JC, Friedmann T . A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA 1994; 91: 9564–9568.
Gossen M, Bujard H . Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551.
Shockett P, Difilippantonio M, Hellman N, Schatz DG . A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA 1995; 92: 6522–6526.
Yao F, Svensjo T, Winkler T, Lu M, Eriksson C, Eriksson E . Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther 1998; 9: 1939–1950.
Hillen W, Berens C . Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu Rev Microbiol 1994; 48: 345–369.
Carrondo MJ, Merten OW, Haury M, Alves PM, Coroadinha AS . Impact of retroviral vector components stoichiometry on packaging cell lines: effects on productivity and vector quality. Hum Gene Ther 2008; 19: 199–210.
Yap MW, Kingsman SM, Kingsman AJ . Effects of stoichiometry of retroviral components on virus production. J Gen Virol 2000; 81: 2195–2202.
Farley DC, Iqball S, Smith JC, Miskin JE, Kingsman SM, Mitrophanous KA . Factors that influence VSV-G pseudotyping and transduction efficiency of lentiviral vectors-in vitro and in vivo implications. J Gene Med 2007; 9: 345–356.
McGrew MJ, Sherman A, Ellard FM, Lillico SG, Gilhooley HJ, Kingsman AJ et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep 2004; 5: 728–733.
Rohll JB, Mitrophanous KA, Martin-Rendon E, Ellard FM, Radcliffe PA, Mazarakis ND et al. Design, production, safety, evaluation, and clinical applications of nonprimate lentiviral vectors. Methods Enzymol 2002; 346: 466–500.
Miskin J, Chipchase D, Rohll J, Beard G, Wardell T, Angell D et al. A replication competent lentivirus (RCL) assay for equine infectious anaemia virus (EIAV)-based lentiviral vectors. Gene Therapy 2006; 13: 196–205.
Arnold BA, Hepler RW, Keller PM . One-step fluorescent probe product-enhanced reverse transcriptase assay. Biotechniques 1998; 25: 98–106.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, H., Leroux-Carlucci, M., Sion, C. et al. Development of inducible EIAV-based lentiviral vector packaging and producer cell lines. Gene Ther 16, 805–814 (2009). https://doi.org/10.1038/gt.2009.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.20
Keywords
This article is cited by
-
Optogenetic Tractography for anatomo-functional characterization of cortico-subcortical neural circuits in non-human primates
Scientific Reports (2018)
-
Production of lentiviral vectors
Molecular Therapy - Methods & Clinical Development (2016)
-
Construction of stable packaging cell lines for clinical lentiviral vector production
Scientific Reports (2015)