Abstract
Purpose
Choroidal thickness (CT) measurements are typically obtained from manual segmentation of optical coherence tomography (OCT) B-scans. This method is time-consuming. We aimed to describe a novel and faster technique to obtain CT measurements.
Patients and methods
In a prospective cohort study of 200 healthy eyes, Spectral-Domain OCT with enhanced depth imaging were performed with the Spectralis OCT using standardised imaging protocols. The OCT scans were independently graded by reading centre-certified graders. The standard method of manual adjustment of segmentation boundaries was performed. The new method consisted of adjusting the lower segmentation line to the choroid-scleral boundary to generate the combined choroid-retina thickness, and subtracting the original retinal thickness (RT) from it to measure CT. Mean CT in the respective Early Treatment Diabetic Retinopathy Study (ETDRS) subfields was measured via the two methods, and were compared with intraclass correlation coefficients (ICC) and Bland–Altman plots.
Results
The mean central subfield CT was 324.4 μm using the original method, compared with 328.8 μm using the new method, with a mean difference of 4.5 μm (range: −14.0 to +4.0 μm; P<0.001), and ICC for agreement of 0.9996 (P<0.001). Similar comparability was achieved for mean CT across other ETDRS subfields, with mean differences ranging from 2.4 to 3.7 μm, and ICCs ranging from 0.9993 to 0.9995 (all P<0.001).
Conclusions
Mean CT can be measured by subtracting the original RT from the combined choroid-retina thickness. Only one segmentation line needs to be adjusted, instead of two, reducing time required for segmentation. This method is faster and reliable.
Similar content being viewed by others
Introduction
The choroid plays important roles in the normal physiology of the eye, including the provision of metabolic support to the retinal pigment epithelium (RPE).1 It is also known to play important roles in the pathophysiology of various retinal diseases, including central serous chorioretinopathy,2, 3 age-related macular degeneration,4, 5 polypoidal choroidal vasculopathy,5 diabetic retinopathy,6 and pathological myopia.7, 8
Optical coherence tomography (OCT) using enhanced depth imaging (EDI) has been used in many studies to measure choroidal thickness in normal populations and in eyes with ocular diseases.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 It has been postulated that variations in choroidal thickness may indicate the presence of retinal diseases, and may be used to monitor its prognosis and treatment progress.9, 10, 11, 12
In some commonly used Spectral-Domain OCT devices, such as the Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany), automated segmentation of the choroidal boundaries are currently unavailable on the in-built software. As a result, choroidal thickness measurements from these scans need to be obtained after manually segmenting the choroid, that is, to manually label the choroidal boundaries using the in-built OCT software.9, 10, 11, 12, 24, 25, 26, 27 The lower segmentation line, which originally delineates the lower border of the RPE-Bruch’s membrane complex, has to be moved down to the choroid-scleral boundary. The upper segmentation line that is originally positioned at the internal limiting membrane is then moved down to the lower border of the RPE-Bruch’s membrane complex. This process is repeated for every OCT B-scan, and is time-consuming and tedious. Its application is argued to be limited for large volume scans and large population studies, though it is reported to have good reproducibility. In most studies, however, the manual quantification of choroidal thickness is performed at the fovea or at specific points in the superior, inferior, temporal, and nasal regions around the fovea (ie, point thickness measurements).13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 This is subjected to inaccuracies due to focal thickening or thinning of the choroid, and irregularities of the choroid-scleral boundary.
Although automated and semi-automated segmentation of the choroid is now possible, many techniques have not entered commercial use and are subjected to various limitations.28, 29, 30, 31, 32, 33, 34, 35, 36 There are also reports that mean choroidal thickness measurements between automated segmentations and manual segmentations on OCT devices may differ considerably.12 Therefore, the manual adjustment of segmentation lines to measure mean choroidal thickness remains relevant and necessary in many quantitative studies of the choroid.
In this study, we aimed to describe a novel and faster method of obtaining choroidal thickness measurements on OCT and to compare this with standard manual grading of the choroidal boundaries.
Methods
This is a prospective cohort study which was performed at the National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore. Two hundred eyes from 100 healthy participants were imaged with Spectral-Domain OCT (SD-OCT). All participants were examined by a trained ophthalmologist (CST) to exclude ocular pathology. Participants with ocular disease or previous ocular surgery were excluded. Myopia was not an exclusion criterion in this study. This study was approved by the Institutional Review Board of the National Healthcare Group, and conformed to the tenets of the Declaration of Helsinki. All participants gave written and informed consent prior to enrolment into the study.
Ocular imaging protocol
SD-OCT with EDI was performed under standardised mesopic lighting conditions. The Spectralis OCT (Heidelberg Engineering) was used with a standardised imaging protocol. A 31-line horizontal raster scan (30o × 25o, 9.2 mm × 7.6 mm) that was fovea-centred was performed. Each OCT B-scan had 25–35 frames averaged to improve the image quality. All OCT scans were reviewed by a fellowship-trained retinal specialist (CST) to ensure that the choroid-scleral boundary on every OCT B scan could be visualised and that the scans were of sufficient clarity.
Axial length was measured using the IOL Master (Carl Zeiss Meditec, Dublin, CA, USA). Refractive error and keratometry were measured using the Canon RK-F1 full autorefractor-keratometer (Canon Inc., Tokyo, Japan).
Obtaining choroidal thickness
Manual adjustment of the segmentation lines on each OCT B-scan were independently carried out by trained and experienced reading centre-certified graders using proprietary OCT viewing software (Heidelberg Eye Explorer version 1.7.1.0). In cases where large discrepancies in measurement between graders occurred, the gradings were adjudicated by the senior investigator.
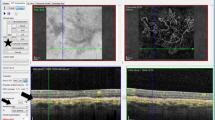
In the original method, which has previously been described,9, 10, 12 the two segmentation lines that originally delineate the upper and lower boundaries of the retina (Figure 1a) were adjusted to the choroid (Figures 1b and c). The lower segmentation line that originally corresponded to the lower border of the RPE-Bruch’s membrane complex was moved down to the choroid-scleral boundary (Figure 1b). The upper segmentation line that originally corresponded to the internal limiting membrane was subsequently moved down to the lower border of the RPE-Bruch’s membrane complex (Figure 1c). It was ensured that the scan was centred on the fovea. The software subsequently calculated the mean choroidal thickness in each Early Treatment Diabetic Retinopathy Study (ETDRS) subfield.
Position of the automated segmentation lines. The letter ‘m’ indicates that the position of the segmentation line has been manually adjusted. (a) At the internal limiting membrane and the RPE-Bruch’s membrane complex (retinal thickness). (b) At the internal limiting membrane and the choroid-scleral boundary (combined choroid-retina thickness). (c) At the RPE-Bruch’s membrane complex and the choroid-scleral boundary (choroidal thickness).
In the new method used in this study, only the lower segmentation line was moved down to the choroid-scleral junction (Figure 1b). The upper segmentation line was left unadjusted at the internal limiting membrane. This generated a map with the combined choroid-retina thickness values. The mean choroidal thickness measurements were then obtained by subtracting the original mean RT from the combined choroid-retina thickness in each ETDRS subfield. The RT measurements were obtained from the original (unadjusted) OCT scans (Figure 1a).
In regions where the boundary was indistinct or uncertain, reference was made to the adjacent areas of the choroid–scleral boundary and to the superior and inferior OCT B-scans to determine the variation of the choroid topography horizontally and vertically.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to assess normality of results. Differences in the mean choroidal thickness measurements from the two methods were analysed using paired t-tests. Intraclass correlation coefficients (ICC) and Bland–Altman plots were used to assess the inter-method agreement. ICC was used to establish the inter-grader agreement for manual grading of choroidal thicknesses on the Spectralis.
Results
The mean age of the participants was 22.5 years (range: 21 to 33, SD±1.8), with 59.0% male and 41.0% female. The mean spherical equivalent was −4.02 D (range: 0.50 to −10.88 D, SD±2.8). The mean axial length was 25.44 mm (range: 22.04 to 29.10 mm, SD±1.4).
Comparison of choroidal thickness in the central ETDRS subfield
The mean central subfield choroidal thickness was 324.4 μm using the original method, compared with 328.8 μm using the new method (Table 1), with a mean difference of 4.5 μm (P<0.001). The differences between both measurements (choroidal thickness obtained via original method compared with that obtained via new method) ranged from −14.0 μm to +4.0 μm. The ICC for inter-method agreement was 0.9996 (P<0.001). The difference in mean choroidal thickness measurements between the two methods is illustrated in the Bland–Altman plot in Figure 2.
Comparison of choroidal thicknesses in other ETDRS subfields
Similar observations were observed for the other ETDRS subfields, as illustrated in Table 1. The mean differences in choroidal thickness between the two methods in the other ETDRS subfields ranged from 2.4 to 3.7 μm (all P<0.001). The corresponding ICCs for inter-method agreement ranged from 0.9993 to 0.9995 (all P<0.001).
Other observations
The refractive error and axial length did not influence the magnitude of differences in mean choroidal thickness measurements between the two methods.
The manual grading of choroidal thicknesses by the graders were very consistent and repeatable, with an inter-grader ICC of 0.989.
Discussion
This study has described a novel and faster method of measuring mean choroidal thickness on OCT devices where automated segmentation algorithms are unavailable. The mean choroidal thickness can be obtained indirectly by subtracting the original RT from the combined choroid-retina thickness, which is measured by adjusting only one segmentation line down to the choroid-scleral boundary. This is faster than adjusting two segmentation lines to measure the mean choroidal thickness directly in the original method.
The differences in the mean choroidal thickness measurements between the two methods were very small (2.4 to 4.5 μm), and the ICCs for inter-method agreement were good (>0.999). This difference in the measurement is likely to be clinically insignificant. A study has reported that interobserver variability in subfoveal choroidal thickness measurements may result in differences of up to 32 μm.16 Furthermore, this difference is also much smaller than the reported diurnal variation of choroidal thickness.37 Studies have reported significant diurnal variation in subfoveal choroidal thickness among healthy adults, and the amplitude (difference between the maximum and minimum choroidal thickness) ranged from 3 to 67 μm.37
The small differences in the mean choroidal thickness and good inter-method agreement are as such, because the process of determining the outer choroidal boundary (choroid-scleral boundary) is common to both methods. The outer choroidal boundary is more difficult to visualise and delineate, owing to the signal falloff and decreased penetration at that depth. In contrast, the inner choroidal boundary (RPE-Bruch’s membrane complex), which needs to be delineated only in the original method, is easier to visualise because of the high contrast of this layer compared with the surrounding structures. Interestingly, the greatest difference in the mean choroidal thickness measurement was in the central ETDRS subfield, though this difference is likely to be negligible.
To the best of our knowledge, there are no other attempts to simplify the process of manually segmenting the choroid to obtain the mean choroidal thickness. Though the time taken to obtain the choroidal thickness measurements were not formally recorded and compared between both methods, eliminating half of the number of segmentation lines to be adjusted in the new method decreases the time needed to manually segment the choroid by approximately half.
Ostensibly, another method to simplify the manual measurements of choroidal thickness would be to move the upper segmentation line that corresponds to the internal limiting membrane to the choroid-scleral interface directly, and to leave the lower segmentation line at the lower border of the RPE-Bruch’s membrane complex. However, the in-built Heidelberg Eye Explorer (HEYEX) software on the Spectralis OCT does not generate values for choroidal thickness if the segmentation lines were to be moved this way. At present, the manual adjustment of segmentation lines to measure mean choroidal thickness remains relevant and necessary in many quantitative studies of the choroid. Many commonly used SD-OCT devices, such as the Spectralis OCT, do not incorporate automated segmentation of the choroidal boundaries. Since the Spectralis OCT is used in many centres, automated segmentation methods are an important area of study.
Although this is not the primary purpose of the study, it should be noted that many automated and semi-automated techniques of choroidal segmentation have not entered commercial use and are subjected to various limitations, despite reports of reasonable agreement between automated analysis and manual segmentation.28, 29, 30, 31, 32, 33, 34, 35, 36 Among others, limitations include (a) sensitivity to noise or missing data, (b) reliance on well-contrasted choroid-scleral boundaries, (c) additional time required to acquire volume scans, (d) need for manual segmentation and training data to construct training images for the segmentation model, and (e) unknown performance in retinal and choroidal diseases. In addition, it has been reported that mean choroidal thickness measurements between automated segmentations from swept-source OCT and manual segmentations on the Spectralis OCT may differ considerably by between 42.1 and 67.2 μm.12 This difference was reduced, but not eliminated, by manual adjustment of segmentation boundaries by trained graders. These discrepancies are likely related to differences in segmentation algorithms, and other device-related factors such as performance, wavelength, resolution, axial magnification, spacing between OCT-B scans, or signal averaging.12 The identification and demarcation of the two boundaries that delineate the choroid, particularly the detection of the choroid-scleral boundary, remains challenging due to the contrast and the non-uniform nature of the vessels. All these suggest that there is still a role for manual grading of choroidal thickness until the technology for automated measurements of choroidal thickness matures.
The strengths of this study include the use of standardised imaging protocols to obtain the OCT scans, and trained reading centre-certified graders to define the choroid-scleral boundaries. The reliability of the manual grading is demonstrated by the high ICC for inter-grader agreement.
Among other limitations, both methods of grading are manual. Even the new method is still relatively time-consuming, and is subjected to grading bias. The participants are also young and healthy, and eyes with pathology other than myopia were excluded. This relative homogeneity in the study population may have contributed to the small differences in mean choroidal thickness and good agreement. A potential consideration, therefore, is that the findings in this paper may not extend to pathological eyes. Nonetheless, the range of choroidal thickness measurements in this study is large – 96 to 624 μm. Furthermore, it is likely that similar to healthy eyes, the time needed to manually measure the choroidal thicknesses of pathological eyes will be decreased if only one segmentation line needs to be adjusted on each OCT B-scan instead of two.
In summary, mean choroidal thickness can be measured by subtracting the original RT from the combined choroid-retina thickness. Only one segmentation line needs to be adjusted on each OCT B-scan, instead of two, which decreases the manual segmentation time. This method is faster and reliable.

References
Feldkaemper MP, Wang HY, Schaeffel F . Changes in retinal and choroidal gene expression during development of refractive errors in chicks. Invest Ophthalmol Vis Sci 2000; 41: 1623–1628.
Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T . Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina 2011; 31: 1603–1608.
Pryds A, Larsen M . Choroidal thickness following extrafoveal photodynamic treatment with verteporfin in patients with central serous chorioretinopathy. Acta Ophthalmol 2012; 90: 738–743.
Manjunath V, Goren J, Fujimoto JG, Duker JS . Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol 2011; 152: 663–668.
Chung SE, Kang SW, Lee JH, Kim YT . Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011; 118: 840–845.
Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS . Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 2012; 32: 563–568.
Coscas G, Zhou Q, Coscas F, Zucchiatti I, Rispoli M, Uzzan J et al. Choroid thickness measurement with RTVue optical coherence tomography in emmetropic eyes, mildly myopic eyes, and highly myopic eyes. Eur J Ophthalmol 2012; 22: 992–1000.
Spaide RF, Koizumi H, Pozzoni MC . Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008; 146: 496–500.
Tan CS, Cheong KX . Macular choroidal thicknesses in healthy adults—relationship with ocular and demographic factors. Invest Ophthalmol Vis Sci 2014; 55: 6452–6458.
Tan CS, Cheong KX, Lim LW, Li KZ . Topographic variation of choroidal and retinal thicknesses at the macula in healthy adults. Br J Ophthalmol 2014; 98: 339–344.
Tan CS, Ngo WK, Cheong KX . Comparison of choroidal thicknesses using swept source and spectral domain optical coherence tomography in diseased and normal eyes. Br J Ophthalmol 2015; 99: 354–358.
Tan CS, Cheong KX, Lim LW, Sadda SR . Comparison of macular choroidal thicknesses from swept source and spectral domain optical coherence tomography. Br J Ophthalmol 2015; 100: 995–999.
Margolis R, Spaide RF . A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 2009; 147: 811–815.
Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y . Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci 2010; 51: 2173–2176.
Ikuno Y, Maruko I, Yasuno Y, Miura M, Sekiryu T, Nishida K et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 5536–5540.
Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L . Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 2267–2271.
Chhablani J, Barteselli G, Wang H, El-Emam S, Kozak I, Doede AL . Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53: 2274–2280.
Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF . Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 2009; 148: 445–450.
Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM . The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol 2013; 155: 314–319.
Ding X, Li J, Zeng J, Ma W, Liu R, Li T et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci 2011; 52: 9555–9560.
Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S, Shiraga F . Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol 2012; 56: 230–235.
Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology 2013; 120: 175–180.
Li XQ, Larsen M, Munch IC . Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci 2011; 52: 8438–8441.
Shin JW, Shin YU, Lee BR . Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography. Ophthalmology 2012; 119: 1017–1023.
Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K et al. Macular choroidal thickness and volume in normal subjects measured by swept source optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 4971–4978.
Ouyang Y, Heussen FM, Mokwa N, Walsh AC, Durbin MK, Keane PA et al. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 7019–7026.
Tan CS, Lim LW, Chow VS, Chay IW, Tan S, Cheong KX et al. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci 2016; 57: OCT224–234.
Alonso-Caneiro D, Read SA, Collins MJ . Automatic segmentation of choroidal thickness in optical coherence tomography. Biomed Opt Express 2013; 4: 2795–2812.
Tian J, Marziliano P, Baskaran M, Tun TA, Aung T . Automatic segmentation of the choroid in enhanced depth imaging optical coherence tomography images. Biomed Opt Express 2013; 4: 397–411.
Danesh H, Kafieh R, Rabbani H, Hajizadeh F . Segmentation of choroidal boundary in enhanced depth imaging OCTs using a multiresolution texture based modeling in graph cuts. Comput Math Methods Med 2014; 2014: 479268.
Lu H, Boonarpha N, Kwong MT, Zheng Y . Automated segmentation of the choroid in retinal optical coherence tomography images. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 5869–5872.
Hu Z, Wu X, Ouyang Y, Sadda SR . Semiautomated segmentation of the choroid in spectral-domain optical coherence tomography volume scans. Invest Ophthalmol Vis Sci 2013; 54: 1722–1729.
Zhang L, Lee K, Niemeijer M, Mullins RF, Sonka M, Abràmoff MD . Automated segmentation of the choroid from clinical SD-OCT. Invest Ophthalmol Vis Sci 2012; 53: 7510–7519.
Duan L, Hong YJ, Yasuno Y . Automated segmentation and characterization of choroidal vessels in high-penetration optical coherence tomography. Opt Express 2013; 21: 15787–15808.
Kajic V, Esmaeelpour M, Povazay B, Marshall D, Rosin PL, Drexler W . Automated choroidal segmentation of 1060 nm OCT in healthy and pathologic eyes using a statistical model. Biomed Opt Express 2012; 3: 86–103.
Gupta P, Jing T, Marziliano P, Cheung CY, Baskaran M, Lamoureux EL et al. Distribution and determinants of choroidal thickness and volume using automated segmentation software in a population-based study. Am J Ophthalmol 2015; 159: 293–301.
Tan CS, Ouyang Y, Ruiz H, Sadda SR . Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53: 261–266.
Acknowledgements
Drs Cheong, Lim and Li do not receive funding. Dr Tan receives research funding from the National Medical Research Council Transition Award (Code: NMRC/TA/0039/2015) and the National Healthcare Group (Singapore) Clinician Scientist Career Scheme Grant (Code: CSCS/12005). Dr Tan also receives conference support from Bayer (South East Asia) Pte. Ltd., Heidelberg Engineering (Heidelberg, Germany), and Novartis (Singapore).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheong, K., Lim, L., Li, K. et al. A novel and faster method of manual grading to measure choroidal thickness using optical coherence tomography. Eye 32, 433–438 (2018). https://doi.org/10.1038/eye.2017.210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.210
This article is cited by
-
Exosomal miR-184 in the aqueous humor of patients with central serous chorioretinopathy: a potential diagnostic and prognostic biomarker
Journal of Nanobiotechnology (2023)