Abstract
Opioid analgesics remain the first choice for the treatment of moderate to severe pain, but they are also notorious for their respiratory depression and addictive effects. This study focused on the pharmacology of a novel opioid receptor mixed agonist DPI-125 and attempted to elucidate the relationship between the δ-, μ- and κ-receptor potency ratio and respiratory depression and abuse liability. Five diarylmethylpiperazine compounds (DPI-125, DPI-3290, DPI-130, KUST202 and KUST13T02) were selected for this study. PKA fluorescence redistribution assays in CHO cells individually expressing δ-, μ- or κ-receptors were used to measure the agonist potency. The respiratory safety profiles were estimated in rats by the ratio of ED50 (pCO2 increase)/ED50 (antinociception). The abuse liability of DPI-125 was evaluated with a self-administration model in rhesus monkeys. The observed agonist potencies of DPI-125 for δ-, μ- and κ-opioid receptors were 4.29±0.36, 11.10±3.04, and 16.57±4.14 nmol/L, respectively. The other four compounds were also mixed agonists with varying potencies. DPI-125 exhibited a high respiratory safety profile, clearly related to its high δ-receptor potency. The ratio of the EC50 potencies for the μ- and δ-receptors was found to be positively correlated with the respiratory safety ratio. DPI-125 has similar potencies for μ- and κ-receptors, which is likely the reason for its reduced abuse potential. Our results demonstrate that the opioid receptor mixed agonist DPI-125 is safer and less addictive than traditional μ-agonist analgesics. These findings suggest that the development of δ>μ∼κ opioid receptor mixed agonists is feasible, and such compounds could represent a promising class of potent analgesics with wider therapeutic windows.
Similar content being viewed by others
Introduction
Opioid receptors (ORs) include μ, δ, κ and opioid receptor-like 1 (ORL-1) subtypes. This receptor family plays a key role in the sensing of pain, thus drawing tremendous attention to their functional and structural details1,2,3,4,5,6,7. Small-molecule μ-OR agonists remain the first choice for the clinical treatment of moderate to severe pain. Unfortunately, μ-agonists are notorious for their life-threatening and/or debilitating adverse effects, including respiratory depression, physical dependence, euphoric addiction, vomiting, constipation and urinary retention8. All of the above side effects are known to be primarily mediated through μ-ORs. Although substantial advances have been achieved in our understanding of the biology of ORs, safer analgesics are still lacking, resulting in patients being under-dosed or deprived of treatment altogether.
The discoveries of the systemically active δ-antagonist naltrindole (NTI)9 and the δ-agonists BW373U8610 and SNC8011 have advanced our understanding of the functions of δ-ORs in vivo over the past two decades12. Initial studies with small-molecule δ-ligands indicated that δ-ORs mediate milder analgesic effects than morphine13,14 and cause convulsive symptoms in rodents15. The δ-agonists BW373U86, SNC80 and AZD2327 were shown to lack addictive effects in rodents and monkeys16,17,18. Since then, investigators have continued to discover potential therapeutic uses for δ-agonists in depression19, Parkinson's disease20, cardioprotection21, neuroprotection22 and overactive bladder23. Some of these indications are currently under clinical investigation.
Given that endogenous opioids, which have mixed receptor effects, do not exhibit the deleterious effects of μ-OR agonists, there have been numerous efforts since the 1990s to demonstrate the therapeutic implications of targeting δ- and μ-ORs simultaneously. One of these efforts attempted to activate both δ- and μ-ORs to reduce the side effects of opioid analgesics. O'Neill et al24 reported antagonistic modulation between the δ-agonist BW373U86 and the μ-agonist fentanyl. When they are administered separately, BW373U86 causes convulsive effects and fentanyl causes muscle rigidity in mice, but their co-administration significantly reduced these side effects. Su et al25 further reported that δ-OR ligands reversed the life-threatening respiratory depression induced by alfentanil without interfering with its analgesic activity. On the other hand, κ-agonists are known to induce dysphoria, aversion and other effects26 that are often opposite to the euphoric effects of μ-agonists27,28. κ-Agonists have been shown to counteract reward behavior in conditioned place preference tests29, inhibit the dopamine release in the nucleus accumbens induced by heroin self-administration30, and decrease the rate of fentanyl self-administration31.
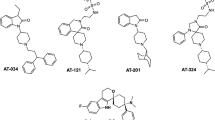
These results sparked intense efforts in the drug discovery community to develop analgesic mixed agonists. Mixed μ-agonist/κ-agonist compounds have been reported to have less abuse liability32,33. Our contribution in these efforts was the discovery of an analgesic diarylmethylpiperazine mixed δ-agonist/μ-agonist, DPI-329034,35,36 (Figure 1). DPI-3290 demonstrated reduced respiratory depression compared with morphine, suggesting that a mixed δ-agonist/μ-agonist could be a safer clinical choice than currently available options.
Diarylmethylpiperazine opioid receptor mixed agonists used in this study.
Following this work, we carried out in-depth structure-activity relationship (SAR) studies on DPI-3290 analogs and discovered another diarylmethylpiperazine compound, DPI-125 (Figure 1), with a similarly high respiratory safety profile. Our results indicated that the κ-OR activity of these compounds also played an important role in their in vivo effect profiles. These compounds are in fact δ-, μ- and κ-OR mixed agonists, a class combining both δ-agonist/μ-agonist and μ-agonist/κ-agonist effects.
Herein, we report our findings on the pharmacology of diarylmethylpiperazine δ-, μ- and κ-OR mixed agonists. Five compounds, DPI-12536,37, DPI-3290, DPI-13036,38, KUST20238 and KUST13T02, were selected for this study (Figure 1). These compounds demonstrate various levels of mixed δ-, μ-, κ-OR activities. Compounds with higher δ-OR potency than μ-OR potency were safer in terms of their propensity to cause respiratory depression, while compounds with relatively high and balanced μ- and κ-OR activity levels exhibited lower addiction liability. Our results demonstrate that DPI-125 is safer than classical μ-agonists in terms of both respiratory depression and addictive effects and suggest that δ-, μ-, κ-OR mixed agonists may represent a promising avenue to effective analgesia with reduced respiratory depression and abuse potential.
Materials and methods
Animals
Male albino Wistar Hannover rats (Harlan, Madison, WI, USA) weighing 200 to 300 g were maintained on a 12-h light/dark cycle (lights on between 7:00 AM and 7:00 PM) and allowed access to food and water ad libitum. A self-administration model in alfentanil-trained and alfentanil-maintained adult male rhesus monkeys39,40,41 was used to evaluate the reinforcing effects of DPI-125 in Prof James H Woods' laboratory at the University of Michigan; these experiments were organized through the Drug Evaluation Committee of the National Institute on Drug Abuse (NIDA) College on the Problems of Drug Dependence (CPDD) (see supplementary information). All experimental procedures were conducted in accordance with guidelines for the use of experimental animals and were approved by the local ethics committee and the Institutional Review Committee on Animal Care and Use.
Cell lines
Recombinant CHO-K1 cells stably expressing human μ-OR and the catalytic domain of human Protein Kinase A (PKAcat) fused to the N-terminus of enhanced green fluorescent protein (eGFP), known as CHO-PKAcat-eGFP/μ-OR cells, were purchased from ThermoFisher (Waltham, MA, USA). PKA Gs- or Gi/o-coupled GPCR redistribution cell lines, consisting of CHO-K1 cells stably expressing PKAcat fused to the N-terminus of eGFP (CHO-PKAcat-eGFP) (ThermoFisher, Waltham, MA, USA) and stably transfected with human κ-OR (named CHO-PKAcat-eGFP/κ-OR) or δ-OR (named CHO-PKAcat-eGFP/δ-OR), have previously been established at the Beijing Institute of Pharmacology and Toxicology. Standard methods were used to generate plasmid DNA. Briefly, full-length cDNAs for the wild-type human δ- and κ-opioid receptors were subcloned individually into pcDNA3.1+ plasmids (Invitrogen, Carlsbad, CA, USA) at the BamHI (5′) and EcoRI (3') sites (δ-OR) or at the KpnI (5′) and XhoI (3') sites (κ-OR). Cells were transfected using the reagent Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as described in the reagent supplier's user manual. These three cell lines were used for PKA redistribution assays; they were cultured at 37 °C in F12 medium supplemented with 10% fetal bovine serum and 200 μg/mL geneticin (G418) in a humidified atmosphere of 95% air and 5% CO2.
Materials
[3H]DPDPE, [3H]DAMGO, and [3H]U69593 were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Morphine, fentanyl, naloxone, U69593, U50488, DAMGO, SNC80 and other chemical reagents were purchased from Sigma-Aldrich (St Louis, MO, USA). All diarylmethylpiperazine mixed OR agonists except KUST13T02 were synthesized according to previously reported methods.
For in vitro experiments, samples of each test compound were dissolved in DMSO to a concentration of 1 mmol/L and then diluted to the required concentrations with F12 medium or buffer solution. The highest concentration of the solutions was 10 μmol/L, corresponding to DMSO concentrations of 1% or less. For in vivo experiments, solutions of the test compounds were prepared by dissolving samples in a minimum amount of ethanol, converting the compounds to hydrochlorides by adding a molar equivalent of concentrated hydrochloric acid, completely drying the solutions under nitrogen flow, and dissolving the solid residues in sterilized 5% dextrose solution. All compound solutions were prepared freshly before use.
Synthesis of KUST13T02
In an oven-dried flask, nicotinaldehyde (1, Figure 2, 1.07 g, 10.0 mmol), (2R,5S)-2,5-dimethyl-1-phenethylpiperazine (2, 2.18 g, 10.0 mmol), benzotriazole (BtH, 1.19 g, 10.0 mmol) and toluene (120 mL) were combined. The mixture was stirred under reflux, and the water generated in the reaction was collected by a Dean-Stark trap. After 3 h, the solvent was removed under reduced pressure. The residue was re-dissolved in anhydrous THF under nitrogen and added to a freshly prepared solution of {3-[(tert-butyldimethylsilyl)oxy]phenyl}magnesium bromide (3, 20.0 mmol) in THF at room temperature. After stirring at room temperature for 5 h, the reaction was quenched by adding a saturated aqueous solution of ammonium chloride. THF was removed under reduced pressure. Ethyl acetate was added, and the mixture was filtered. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated. The crude product was purified by flash column chromatography (silica gel, ethyl acetate) to obtain a TBS-protected intermediate (4), which was then dissolved in THF and treated with tetrabutylammonium fluoride (TBAF, 1 mol/L in THF). The mixture was concentrated and the residue was partitioned between ethyl acetate and water. The organic layer was separated, dried over anhydrous magnesium sulfate, and concentrated. The crude product was purified by flash column chromatography (silica gel, ethyl acetate) to obtain KUST13T02 as a white powder (18% yield for two steps). 1H NMR (400 MHz, CDCl3): δ 8.55 (s, 1H), 8.35–8.31 (m, 1H), 7.76 (d, J=8.0 Hz, 1H), 7.18–7.01 (m, 8H), 6.68–6.64 (m, 1H), 6.55–6.50 (m, 1H), 5.09 (bs, 1H), 2.89–2.74 (m, 2H), 2.73–2.58 (m, 3H), 2.56–2.47 (m, 2H), 2.42 (d, J=10.5 Hz, 1H), 2.26 (dd, J=10.5, 9.5 Hz, 1H), 1.87 (dd, J=10.5, 9.5 Hz, 1H), 1.05 (d, J=6.0 Hz, 3H), 0.89 (d, J=6.0 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 157.58, 149.07, 146.76, 140.12, 138.83, 136.81, 129.43, 128.73, 128.52, 126.16, 123.45, 121.02, 117.14, 115.30, 77.45, 63.56, 55.38, 55.24, 53.21, 51.76, 31.60, 28.54, 16.02. HRMS (ESI) calcd. for C26H32N3O+ [M+H]+ 402.2545; found 402.2541.
Synthesis of diarylmethylpiperazine opioid receptor agonist KUST13T02.
Membrane preparation for radio-ligand binding
Membrane preparation was performed as previously described10. The brains of male albino Wistar Hannover rats were rinsed with ice-cold 50 mmol/L Tris-HCl buffer (pH 7.4, 25 °C) containing the following protease inhibitors: 50 μg/mL soybean trypsin inhibitor, 0.1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L EDTA, 10 μg/mL leupeptin, 200 μg/mL bacitracin, and 0.5 μg/mL aprotinin. Brains were minced with scissors and homogenized in 10 volumes/g wet weight of ice-cold 50 mmol/L Tris-HCl buffer containing protease inhibitors, and then centrifuged at 6000×g for 15 min at 4 °C. The resulting supernatant was centrifuged at 41 000×g for 30 min, and the membrane pellets were re-suspended in 10 volumes/g wet weight of 10 mmol/L Tris-sucrose buffer and sonicated with a Polytron tissue grinder (10 s, low speed). The homogenate was centrifuged again for 30 min at 41 000×g and 4 °C. The resulting membrane pellet was re-suspended in 50 mmol/L Tris buffer with protease inhibitors to a final protein concentration that ranged from 40 to 50 μg/mL. These membrane fractions were frozen under liquid N2 and stored at -80 °C before use in radio-ligand binding studies. Protein determination was performed as previously described42.
Radio-ligand receptor binding
Membrane fractions were incubated with [3H]DPDPE (0.1 nmol/L, 50.6 Ci/mmol), [3H]DAMGO (0.1 nmol/L, 50.0 Ci/mmol), or [3H]U69593 (0.1 nmol/L, 41.4 Ci/mmol) for binding to δ-, μ-, or κ-ORs, respectively. Samples were incubated for 90 min at 25 °C in a total volume of 2.0 mL 10 mmol/L Tris-HCl buffer containing 5 mmol/L MgCl2 and protease inhibitors, with 15–20 μg of protein per tube. The reaction was terminated by rapid filtration through GF/C glass fiber filters (Whatman, Maidstone, UK) using a cell harvester (model M-48R; Brandel Inc, Gaithersburg, MD, USA), followed by two 5-mL rinses with ice-cold 50 mmol/L Tris-HCl buffer. Specific binding was defined as displacement of the radio-ligand by 1.0 μmol/L naloxone. Filters were counted by liquid scintillation spectrometry at an efficiency of 40% to 45%, as determined by external standards. The half-maximal inhibitory concentrations (IC50) were obtained from the competition curves of the radio-ligand receptor binding assays. The apparent binding affinities (Ki) were calculated using the Cheng and Prusoff equation43.
Opioid receptor-mediated PKAcat-eGFP redistribution assays
Cells were inoculated into 96-well plates (approximately 10 000 cells/well) and cultured as described above. After 24 h, the cells were incubated for 15 min in 200 μL F12 medium, F12 medium containing 10 μmol/L forskolin, or F12 medium containing varying concentrations of the test compounds in the presence of 10 μmol/L forskolin. Cells were then fixed with 200 μL/well 4% formaldehyde for 20 min, followed by three 200-μL rinses with PBS. Finally, 100 μL PBS containing 1 μmol/L Hoechst33342 was added for nuclear staining. Cytoplasmic fluorescence spot formation was measured using the IN Cell Analyzer 2000 Cellular Imaging and Analysis System (GE, Fairfield, CT, USA). Activity (%) was calculated relative to the positive (forskolin only) and negative (F12 medium) controls. The concentration-response data were fitted to sigmoidal curves to determine the 50% effective concentration (EC50) values and the maximal effects (Emax).

I: Intensity; BI: Background intensity; TA: Total Area
Selective standard agonists (SNC80 for δ-OR, DAMGO for μ-OR and (±)U50488 for κ-OR) were used to normalize the Emax and EC50 values for all test compounds for each receptor type. The Emax values obtained for SNC80, DAMGO and (±)U50488 were set at 100% for their respective receptors. These values were then used to reconstruct and normalize the concentration-response curves and to obtain the EC50 values for all test compounds and the Emax values for the five mixed opioid analogs.
Blood gas and antinociceptive studies
Male albino Wistar Hannover rats weighing 200 to 300 g were anesthetized with 2% isoflurane in a 30% O2 and 70% N2O vehicle. The femoral artery and external jugular vein were cannulated with silastic tubing. Anesthetic gases were then discontinued, and the rats were allowed to recover in a plastic restrainer for 60 min before administration of the test compounds. Arterial blood gasses and antinociceptive responses were measured simultaneously.
Blood gas measurements were conducted as previously described35. Briefly, every five minutes after intravenous administration of a test compound, 0.15 mL arterial blood was drawn into a syringe with heparin. The syringe was capped, and the blood was analyzed immediately (Ph/Gas analyzer Synthesis 25 model; Instrumentation Laboratory, Lexington, MA, USA). The ED50 pCO2was defined as the dose of a compound that produced a 50% increase in the plasma pCO2 level.
Analgesic effects were measured soon after blood was drawn, using a modified Haffner's tail-pinch protocol with a fixed pressure developed by Takagi et al44. Briefly, straight Blalock artery clamps (7 cm long, branches 2.7 cm long) producing approximately 1100 grams of pressure at the center point of the branches (0.8 cm from the tip, pressure measured by a spring scale) were used for this test. The test was performed with the rat in a plastic cage. A clamp was placed on the tail (1 inch from the tip) and was left in place until an escape response occurred (ie, tail-flick, clamp-biting or vocalization) or for a maximum time of 20 s. The escape response time was recorded using a stopwatch. The basal response time for each rat was determined to be approximately 2 s. Rats with unusual response times were excluded from the experiment. To evaluate analgesic effects, the response time for each individual rat was measured and recorded before and after the injection (iv) of the test compounds. The potency of compounds was determined based on the recorded response times after compound administration, rather than the partial analgesia (response time 2 to 6 s) and complete analgesia (response time >6 s) classification adopted by Takagi et al44. The latency of the escape response was calculated and converted into a percent maximal possible effect score, as described below. The determination of dose-response relationships and statistical analyses were performed using the Prism program.
Maximum percent effect (MPE%)=(response time after compound administration–basal response time)/(maximum time– basal response time)×100%. (Maximum time=20 s)
The analgesic ED50 values of morphine and fentanyl were determined by this protocol in our previous work34,35 and found to be similar to their known antinociceptive potency in rats, thus confirming the validity of the protocol.
Data analysis and statistics
Pharmacological data in vivo were analyzed by linear regression of the linear portion of the dose-response curves, and the competition receptor binding data and concentration-response data in PKAcat-eGFP redistribution assays were fitted by nonlinear regression analysis. Correlations of in vivo effects (ED50 for antinociception and respiratory depression) with measures of receptor activity (EC50 for potency and Ki for binding affinity) were also analyzed by linear regression. Data were analyzed using the computer program Prism (GraphPad Software Inc, San Diego, CA, USA). All data in this study except ratio values are expressed as the mean±SEM.
Results
Binding affinity of opioid receptor triple agonists to opioid receptor subtypes
Following our reports that outlined the synthesis and pharmacology of the first nonpeptidic δ-OR agonist BW373U8610,13,14,15,16,45, DPI-125, DPI-3290, DPI-130, KUST202 and KUST13T02 were identified in a structure-activity relationship study and subjected to further pharmacological testing. The binding affinities (Ki values) of these compounds are summarized in Table 1. Similar to our previous results34,35, these compounds exhibited mixed δ-, μ- and κ-OR ligand activities. Although there were slight differences among the different OR types, all compounds showed high affinities for δ-, μ-, and κ-ORs, with Ki values of approximately 1 nmol/L. The only exceptions were DPI-130, which had a far lower affinity for κ-ORs (Ki 21.8 nmol/L), and KUST13T02, which had a higher affinity for μ-ORs (Ki 0.26 nmol/L) and lower affinity for δ-ORs (Ki 16.5 nmol/L).
Development of PKAcat-eGFP redistribution assay for OR activation
ORs belong to the family of G-protein-coupled receptors (GPCRs)46,47,48. Upon the activation of ORs by opioids, GTP binds to pre-coupled Gα protein and cause the dissociation of the activated GTP-Gα and Gβγ subunits. These subunits then proceed to activate voltage-dependent K+ channels and inactivate Ca++ channels, thus reducing membrane excitability, and reducing cyclic adenosine monophosphate (cAMP) levels by adenylyl cyclase inhibition. The activity of protein kinase A (PKA) is dependent upon intracellular cAMP levels. Using a previously described protocol49,50,51, we used GFP-tagged catalytic subunits (PKAcat-eGFP) to monitor PKA activity as a measure of opioid receptor activation. In the resting inactive state, PKAcat-eGFP forms aggregates inside the cells. Upon activation by increased levels of cAMP, the eGFP-tagged catalytic subunit dissociates from PKA and disperses uniformly throughout the cytoplasm. This redistribution transition can be monitored under fluorescence microscopy and quantified to measure the activity of GPCR-coupled Gs upon activation. Forskolin activates adenylyl cyclase directly to increase cAMP levels and disperse the aggregated fluorescent subunits. The activation of ORs leads to the inhibition of adenylyl cyclase and a decrease in cAMP levels and can therefore be measured by the reappearance of aggregate spots in the presence of forskolin.
Potency of OR triple agonists in the PKAcat-eGFP redistribution assay
PKA is a ubiquitous serine/threonine protein kinase and a major mediator of intracellular cAMP signaling in eukaryotes whose activity is dependent on cellular levels of cAMP. When cytosolic cAMP level increases, two cAMP molecules bind to each PKA regulatory subunit of the R2C2 complex, the regulatory subunits dissociate from the catalytic subunits, and the free catalytic subunits are activated. In this assay, human δ-, μ- and κ-ORs were stably transfected into a GPCR reporter assay cell line for Gs- or Gi/o-coupled receptors that stably expresses the catalytic domain of human PKA (PKAcat) fused to eGFP. Binding of opioid agonists to these ORs causes the activation of the Gi/o complex in intact cells, leading to the inhibition of adenylyl cyclase, a decrease in the formation of cAMP from ATP, and the reassociation of the PKA regulatory and catalytic subunits. This in turn leads to the aggregation of PKAcat-eGFP in cytoplasmic foci. In contrast, cells stimulated with forskolin generate high levels of cAMP, resulting in a diffuse distribution of PKAcat-eGFP in the cytoplasm. The redistribution of PKAcat-eGFP reflects changes in cytoplasmic cAMP concentrations in response to regulation by opioid receptors. This assay is conducted in intact cells rather than prepared membranes and may therefore provide results that are more germane to in vivo conditions.
Due to the heterogeneous distribution of ORs in the brain, comparison with standard agonists of each receptor type to correct for variation is a validated common practice for in vivo pharmacological studies. For in vitro studies on transfected cells, since the observed activity of an agonist can vary depending upon the receptor density on the cell surface, the use of standard agonists is also essential in assessing potency in different cell lines. The standard full agonists SNC80 (δ-OR), DAMGO (μ-OR), and (±)U50488 (κ-OR) increased the aggregation of PKAcat-eGFP in a concentration-dependent manner. The EC50 values and maximal effects (Emax), as shown in Figure 3 and Table 2, are consistent with values reported previously with GTPγ35S] binding assays52. The EC50 values are 6.30, 30.80 and 5.88 nmol/L for SNC80, DAMGO and (±)U50488, respectively (Table 2).
Concentration-dependent activities of PKA redistribution assay. •: CHO-PKAcat-eGFP/δ-OR cell; ▴: CHO-PKAcateGFP/μ-OR cell; ▽: CHO-PKAcat-eGFP/κ-OR cell. (A)OR type selective full agonists; (B)DPI-125; (C) DPI-3290; (D) DPI-130; (E) KUST202; (F) KUST13T02. Concentration-response curves for all compounds were normalized after setting Emax for the standard agonists SNC80, DAMGO and (±)U50488 as 100% at their respective receptors. Each value represents the mean±SEM from at least three independent experiments performed in quadruplicate. The EC50 and Emax values were shown in Table 2.
In general, most of our compounds showed high potency for each receptor, and the Emax values before normalization were greater than 70% of that for the forskolin control. However, the EC50 values were significantly different. The activity of DPI-3290 was consistent with our previous results measuring OR-mediated inhibition of tension development in the vas deferens of mice34. The EC50 values for DPI-3290 for δ-, μ- and κ-opioid receptors were 5.48, 16.27, and 13.29 nmol/L, respectively (Figure 3C, Table 2); these are lower than or similar to values for the corresponding standard agonists. The EC50 values for DPI-125 for δ-, μ- and κ-opioid receptors were 4.29, 11.10 and 16.57 nmol/L (Figure 3B, Table 2), respectively, similar to the activities of DPI-3290. Treatment with DPI-125 caused aggregation of PKAcat-eGFP in the redistribution assays for all three ORs. DPI-130 activities were lowest for κ-ORs, with EC50 values of 2.40, 17.14 and 92.58 nmol/L for δ-, μ- and κ-opioid receptors, respectively (Figure 3D, Table 2).
The percent maximal responses induced by the compounds ranged from 80% to 130% (Table 2). The potency of a compound is proportional to its binding affinity and activation efficacy at a particular receptor and negatively related to the EC50 values of the agonist concentration-response curves from the PKA redistribution assay (ie, lower EC50 values reflect higher potency and vice versa). The potency of DPI-3290 was highest for δ-ORs (EC505.48±0.85 nmol/L), followed by κ-ORs (EC50 13.29±1.93 nmol/L) and μ-ORs (EC50 16.27±2.23 nmol/L) (Figure 3C, Table 2). The difference in potency between κ- and μ-ORs was not significant. The potency of DPI-130 was highest for δ-ORs (EC50 2.40±0.11 nmol/L), intermediate for μ-ORs (EC50 17.14±2.39 nmol/L) and lowest for κ-ORs (EC5092.58±8.75 nmol/L) (Figure 3D, Table 2). Similarly, the potency of DPI-125 was highest for δ-ORs (EC504.29±0.36 nmol/L), intermediate for μ-ORs (EC50 11.10±3.04 nmol/L), and lowest for κ-ORs (EC50 16.57±4.14 nmol/L) (Figure 3B, Table 2). The difference in potency between κ-ORs and μ-ORs was not significant. In contrast, KUST13T02 exhibited potent activity for μ-ORs (EC50 6.54±1.71 nmol/L) but less potent activity for δ-ORs (EC50 20.64±5.44 nmol/L) and much weaker activity for κ-ORs (EC50 74.31±22.2 nmol/L) (Figure 3F, Table 2). KUST202 showed relatively weak activities at all three receptors, with a maximum potency for κ-ORs (EC50 37.29±4.65 nmol/L) (Figure 3E, Table 2).
OR triple agonist-mediated antinociception and hypercapnia in rats
A tail-pinch test was used in this study, as it allows experiments on the antinociception and respiratory depression effects of opioid compounds to be conducted simultaneously in the same animal34,35. This test was reported by Takagi et al44 as a modification of the original Haffner's test53. In our experiments, a straight Blalock artery clamp was placed on the tail one inch from the tip to provide a noxious stimulus. The chosen clamps produced pressures of approximately 1100 g at the center point of the branches. The basal response time to this stimulus was recorded for each animal and was approximately 2 s. The response time increased under the influence of opioids, depending upon the potency of the compound and the dose administered to the animals. The cut-off maximum time was set at 20 s to avoid tissue damage. The maximum percent effect (MPE%) was then calculated, and the determination of dose-response relationships and statistical analyses were performed using the Prism program.
As expected, the tested compounds presented the same characteristics as typical narcotic analgesics because of their strong binding affinity and high activity for μ-ORs. Table 3 shows that the antinociceptive ED50 values of DPI-125 and DPI-3290 were estimated as 0.050±0.005 and 0.050±0.007 mg/kg (iv) in the rat tail-pinch test, much better than that of morphine (ED50 2.01±0.0005 mg/kg) under the same conditions. DPI-130 exhibited slightly weaker antinociceptive effects (ED50 0.08±0.007 mg/kg), while KUST202 showed the weakest effects (ED50 0.16±0.02 mg/kg). KUST13T02 showed the highest potency at μ-ORs in the PKA redistribution assay and potent in vivo antinociception activity (ED50 0.004±0.0003 mg/kg).
The respiratory depression effects of these compounds were also measured simultaneously in conscious laboratory rats (Table 3). Consistent with our previous results35, these compounds produced pCO2 increases like morphine and fentanyl but at doses markedly higher than those necessary for antinociception. The ratio between the respiratory depression and antinociception activities [ED50 (pCO2 increase)/ED50(antinociception), respiratory safety ratio] provides a measure of the safety profile of opioid analgesics. The ED50 values for respiratory depression (pCO2 increase) of DPI-125, DPI-3290, DPI-130, KUST202 and KUST13T02 were 0.72, 0.91, 2.15, 2.2 and 0.034 mg/kg, respectively, which were 14.4-, 18.2-, 26.9-, 13.8- and 8.5-fold higher, respectively, than their ED50 values for antinociception. The safety ratios of morphine and fentanyl are much lower (2.1 and 3.7) than those noted for these novel test compounds.
To gain further insight into the properties of these compounds, we conducted linear regression analyses on the above data. Unsurprisingly, plots of the antinociception ED50 values against the logarithm of potency (logEC50) for μ-ORs in the CHO-PKAcat-eGFP/μ-OR redistribution assay showed a high positive correlation (R2=0.955, P=0.0041 for the regression line) (Figure 4A). However, the correlation between ED50 values for pCO2 increase and logEC50 values for μ-ORs was poor (R2=0.618, P=0.1147 for the regression line) (Figure 4B), suggesting that other factors are involved. It is well accepted that respiratory depression is mediated primarily by μ-ORs. Consistent with previous reports that δ-OR agonists can reverse μ-OR agonist-induced respiratory depression25, these results suggest that δ-ORs play an important role as suppressors of μ-OR agonist-induced respiratory depression.
Correlation of ED50 values of antinociception and respiratory depression with receptor agonist potencies. (A) Correlation between antinociception ED50 values and logarithm of potency (log EC50) at μ-OR. (B) Correlation between respiratory depression ED50 values and logarithm of potency (log EC50) at μ-OR. (C) Correlation between the respiratory safety ratio and the ratio of receptor potency EC50 (μ-OR)/EC50 (δ-OR). Data were shown in Table 3. Safety ratios determined by ED50 values of opioid-mediated hypercapnia divided by ED50 values of opioid-mediated antinociception in conscious laboratory rats. Linear regression was analyzed by the Prism program.
Positive correlation between the μ-/δ-OR EC50 potency ratio and the respiratory safety ratio
It is well known that analgesia and respiratory depression are mainly mediated via μ-ORs. The receptor binding affinities and potencies reported above provide further insight into the in vivo effects of the tested compounds. The OR selectivity of these compounds, ie, the ratios of potency between OR subtypes (μ/δ, κ/δ and μ/κ EC50 ratios), are shown in Table 3 and plotted against the respiratory safety ratios. Figure 4C shows an excellent positive correlation for the safety ratio with the μ/δ EC50 ratio (R2=0.97, P=0.0021 for the regression line) but not with the κ/δ and μ/κ EC50 ratios (data not shown). These results explain why the compounds with a higher potency at δ-ORs than at μ-ORs are safer drugs, suggesting that the safety of opioid analgesics could be improved by combining them with δ-OR agonists or using δ- and μ-OR mixed agonists instead.
On the other hand, Figure 5 shows that both antinociceptive effects (Figure 5A, R2=0.8769, P=0.0191) and respiratory depression (Figure 5B, R2=0.9362, P=0.0070) are correlated with the binding affinity at μ-ORs but not that at δ-ORs or κ-ORs (data not shown). Figure 5C shows that the safety ratio is correlated with the μ/δ affinity ratio (R2=0.8821, P=0.0178). However, the correlation (R2) between antinociception and binding affinity (Figure 5A) is weaker than that between antinociception and μ-OR potency (Figure 4A), and the P-value is also much larger, reflecting a lower significance. Similarly, the observed correlation and significance in the regression analyses were lower for the affinity ratios (Figure 5C) than for the potency ratios (Figure 4C). This suggests that for these mixed agonists receptor potency is a more specific measure of respiratory safety than binding affinity.
Correlation of ED50 values of antinociception and respiratory depression with receptor binding affinities. (A) Correlation between antinociception ED50 values and logarithm of receptor binding affinity (logKi) at μ-OR. (B) Correlation between respiratory depression ED50 values and logarithm of receptor binding affinity (logKi) at μ-OR. (C) Correlation between the respiratory safety ratio and the ratio of receptor binding affinity Ki (μ-OR)/EC50 (δ-OR). Data of receptor binding affinity were shown in Table 1. Safety ratios determined by ED50 values of opioid-mediated hypercapnia divided by ED50 values of opioid-mediated antinociception in conscious laboratory rats. Linear regression was analyzed by the Prism program.
Reinforcing effect of DPI-125 in rhesus monkeys
The reinforcing effects of DPI-125 were evaluated using a self-administration model in alfentanil-trained and alfentanil-maintained adult male rhesus monkeys, which is widely accepted for testing the abuse potential of various substances, including opioids and cocaine39,40,41; these evaluations were performed through the College on the Problems of Drug Dependence (CPDD) (see supplementary information). Four doses of DPI-125 were evaluated in four rhesus monkeys. Each monkey was tested at least twice at each dose. The dose-effect curve (mean±SEM) of DPI-125 self-administration was aggregated across all four monkeys and plotted as a percentage of the alfentanil-maintained response. DPI-125 was self-administered by all four monkeys in the study. The rates of response for DPI-125 were high across a dose range approximately 30-fold higher than that required to engender a contingent response with alfentanil. The maximal rate of response for DPI-125 at 0.01 mg/kg each injection peaked at approximately 70% of the alfentanil maximum positive control. At a higher DPI-125 dose of 0.03 mg/kg each injection, the rate of response dropped close to that of the vehicle control, yielding an inverted U-shaped curve. This comparison indicates that DPI-125 is 30-fold less potent and 30% less effective than alfentanil in terms of reinforcing effects, despite their approximately equal antinociceptive potency in rats54.
Discussion
One objective of the present study was to determine the potency at each OR type for the selected compounds. The availability of three cloned cell lines expressing exclusively δ-, μ-, or κ-ORs made it possible to assess potency without receptor cross-interference, which is otherwise inevitable when using tissue samples. Assessing the potencies of mixed OR agonists for each type of receptor is essential in order to understand receptor signaling crosstalk in vivo. The ranking order of OR subtype potencies for the tested compounds was as follows: DPI-125, δ>κ>μ within a relatively small range; DPI-3290, δ>μ>κ within a relatively small range, similar to DPI-125; DPI-130, δ>μ>κ within a much wider range than DPI-125 and DPI-3290; KUST202, κ>δ>μ, most active for κ-ORs; and KUST13T02, μ>δ>κ, most active for μ-ORs (Figure 3, Table 2). Of note, the profile of KUST13T02 was very different from those of the other four compounds. Thus, these compounds provide a broad spectrum of receptor potencies to correlate with their in vivo pharmacology.
The analgesic effects of opioids are known to be mainly mediated by μ-ORs. The excellent correlation between antinociception (ED50) in rats and receptor potencies (EC50) for μ-ORs (Figure 4A) is consistent with this concept and validates the use of the PKAcat-eGFP fluorescence redistribution imaging analysis to measure the receptor potencies of opioids. Respiratory depression is also mediated by μ-ORs at higher dosages but can be observed only in vivo. μ-ORs located in the rostral ventromedial medulla nucleus of the brain stem are known to be responsible for opioid-induced respiratory depression55,56. The reversal of μ-OR agonist-induced respiratory depression by δ-OR agonists was shown to occur in the central nervous system25. The poor correlation between ED50 values for pCO2 increase and EC50 values for μ-ORs (Figure 4B) suggests that other ORs may be involved in the regulation of respiratory depression. The excellent correlation (Figure 4C) between the ratio of ED50 values for pCO2 increase/ED50 values for antinociception and the μ-/δ-OR EC50 potency ratio confirms the regulatory role of δ-ORs in μ-OR-mediated respiratory depression.
Recent studies of OR heteromers might provide new insights into the mechanisms underlying opioid effects. The formation of OR heteromers has been well described in the literature57,58,59,60,61,62. These heteromers might provide the foundation for various classes of interactions between ORs, resulting in cellular responses different from those associated with a single receptor type63,64,65. However, the physiological significance of OR heteromers remain largely unclear. It is possible that some adverse effects of opioids result from direct interactions between OR subtypes via receptor heteromers, but others result from interactions between neurons with different ORs via neural networks or circuits. Further investigation and evidence are required to understand the link between molecular mechanisms and physiological observations.
Regardless of the molecular details of opioid effects, the present study supports the possibility of developing an opioid analgesic with lower respiratory depression side effects by combining δ-agonist and μ-agonist properties to create opioids with greater potency for δ-ORs than for μ-ORs. This type of opioid can produce analgesic effects via the activation of μ-ORs and simultaneously dampen μ-agonist-induced respiratory depression via its activity at δ-ORs, thus providing effective analgesia with greater safety (Figure 6). The present results suggest the possibility of a ten-fold improvement in the respiratory safety profile compared with that of morphine (Table 3). With the exception of KUST13T02, the tested compounds showed greater potency for δ-ORs than μ-ORs, as well as significant improvements in the safety index, which was 4.05 (8.5/2.1) times higher for KUST13T02 and 12.8 (26.9/2.1) times higher for DPI-130 than for morphine (Table 3). Thus, mixed OR agonists clearly have potential for clinical use.
Hypothetic model of in vivo effect counteraction for mixed agonists with δ>μ∼κ OR potency. Arrows and words represent effects mediated by μ-OR. T-shaped line ⊥ represents negatively regulation. The respiratory depression and addiction adverse effects of μ-OR activation are hypothetically reduced or eliminated by δ- and κ-OR activation, respectively. δ-, μ- and κ-ORs may locate in the same neuron, or in different neurons in the same/different brain areas connected through neural networks or circuit.
Furthermore, the potency ratios between μ-ORs and κ-ORs (Table 3) provide a plausible explanation for the observed reduced abuse potential of some δ-, μ-, and κ-OR mixed agonists. DPI-3290 is known to produce fewer withdrawal syndromes in rats than morphine36. The reinforcing effects of DPI-125 were also found to be far less potent and less effective than those of alfentanil in a rhesus monkey self-administration model39,40,41 (see supplementary information).
It is worth noting that the dose-response curve of reinforcing effects for DPI-125 is similar to the results reported for nalbuphine39, a mixed partial μ-agonist/κ-agonist that was a schedule II analgesic initially and was later removed from the list of controlled substances. When evaluated using the same model as in the present study, the reinforcing effects of nalbuphine occurred in a range of 0.0003 to 0.01 mg/kg each injection, indicating that this drug was approximately 10-fold less potent than alfentanil. The maximum effect reached was only approximately 50%, and the dose-response curve had an inverted U shape. In contrast, the reinforcing effects of traditional μ-agonist analgesics occur at much lower doses39. Alfentanil produces responses in a dose range of 0.00003 to 0.0003 mg/kg each injection. For highly addictive heroin, responses occur at a similar range of 0.00003 to 0.001 mg/kg each injection. The reinforcing effects of morphine are 10-fold less potent than those of alfentanil, which is consistent with its log-order lower analgesic potency compared to alfentanil. Furthermore, morphine is 40-fold less potent than DPI-125 in terms of analgesic potency (Table 3), but its reinforcing effects are approximately equivalent to those of DPI-125. Put together, these results suggest that, although DPI-125 may still be addictive, it has significantly less addiction liability than μ-agonist analgesics relative to its analgesic potency.
μ-ORs are known to mediate the development of physical and psychological dependence. Physical dependence arises from the chronic use of opioids and is precipitated by physical adaptation upon the withdrawal of opioids, whereas psychological dependence results from the euphoric effects of opioids. On the other hand, κ-agonists are known to induce dysphoria and aversive effects26 opposite to the euphoric effects of μ-agonists27. Therefore, a mixed μ-OR/κ-OR agonist with an appropriate potency ratio may provide analgesia with reduced addiction potential. Indeed, κ-OR agonists and mixed μ-OR/κ-OR agonists have been shown to attenuate heroin self-administration in monkeys and rats31,32,33. Similar to the case of respiratory depression, it is also likely that the μ-OR and κ-OR signaling systems counteract each other via neural networks or circuits or through μ/κ-OR heteromers, resulting in a more neutral response (Figure 6).
The compounds investigated in the present study showed a broad range of μ-OR/κ-OR potency ratios (0.088 (KUST13T02), 0.185 (DPI-130), 0.670 (DPI-125), 1.224 (DPI-3290) and 3.406 (KUST202)) (Table 3). Notably, DPI-125 and DPI-3290 have the most balanced potency ratios among these compounds, and they also showed more potent activity for κ-ORs than the other compounds (Table 2), strongly suggesting that the reduced withdrawal syndromes and reinforcing effects associated with these compounds are positively correlated with their κ-OR activity levels.
In addition, convulsion, a common adverse effect of some δ-OR selective agonists, such as BW373U86, appears to not be a problem with DPI-125. When tested in mice, the species known to be most sensitive to δ-OR agonist-induced convulsive effects, the lowest dose producing convulsion was 2–3 mg/kg iv, in one out of ten mice. This dosage is approximately 40-fold higher than the antinociceptive potency of DPI-125 (ED50 0.05 mg/kg iv) and higher than the dosage that induces respiratory depression (ED50 0.72 mg/kg iv). Finally, in all in vivo tests in rats, convulsion effects were not observed after iv administration of dosages that produced complete antinociception effects.
On the other hand, the correlation between agonist potency at each receptor subtype and the safety of the compounds suggests that comparing receptor binding affinities may not be sufficient to predict the safety of δ-, μ-, and κ-OR mixed agonists. In fact, many δ-, μ-, and κ-OR mixed ligands in our compound library did not show improved respiratory safety, which could not be predicted from their receptor binding affinity (not shown). Similarly, a large number of potential δ-, μ-, and κ-OR mixed agonists have been reported in the literature but with only binding affinity data available. Therefore, there is a need to continue searching for better δ>μ∼κ mixed OR agonists based on receptor potency rather than receptor binding affinity.
In summary, the in vitro and in vivo pharmacology of five diarylmethylpiperazine compounds, including DPI-125, was assessed. Cell-based PKA fluorescence redistribution assays were used to measure potency for δ-, μ- and κ-ORs, and tail-pinch antinociception assays and blood gas measurements in rats were used to evaluate the analgesic and respiratory safety profiles. The abuse liability of DPI-125 was also evaluated in a self-administration rhesus monkey model. DPI-125 and the other four compounds are all mixed δ-, μ- and κ-OR agonists with various combinations of potencies. DPI-125 exhibited strong analgesic potency, high respiratory safety and reduced abuse tendency; these effects are presumably related to its strong δ-OR potency and balanced potencies for μ-ORs and κ-ORs. The fact that DPI-125 is safer and less addictive than traditional μ-agonist analgesics suggests that δ>μ∼κ triple OR agonists may have significant clinical potential. In addition, our results emphasize the importance of further investigation of the interactions between OR signaling systems.
Author contribution
Shou-pu YI and Qing-hong KONG designed and performed the research, and wrote the first draft. Jie YU, Ben-qiang CUI, and Ying-fei WANG synthesized and characterized the tested compounds. Yu-lei LI and Chen-ling PAN performed the research. Guan-lin WANG, Pei-lan ZHOU, Li-li WANG, and Gang YU participated in experimental design. Ze-hui GONG, Rui-bin SU, and Gang YU revised and commented on the manuscript. Kwen-jen CHANG and Yue-hai SHEN supervised the project and wrote the manuscript with contributions from all the authors.
References
Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK . Structure of the delta-opioid receptor bound to naltrindole. Nature 2012; 485: 400–4.
Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012; 485: 321–6.
Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 2012; 485: 395–9.
Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012; 485: 327–32.
Fenalti G, Giguere PM, Katritch V, Huang XP, Thompson AA, Cherezov V, et al. Molecular control of delta-opioid receptor signalling. Nature 2014; 506: 191–6.
Huang WJ, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, et al. Structural insights into mu-opioid receptor activation. Nature 2015; 524: 315–21.
Sounier R, Mas C, Steyaert J, Laeremans T, Manglik A, Huang WJ, et al. Propagation of conformational changes during mu-opioid receptor activation. Nature 2015; 524: 375–8.
Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y . Current research on opioid receptor function. Curr Drug Targets 2012; 13: 230–46.
Portoghese PS, Sultana M, Takemori AE . Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol 1988; 146: 185–6.
Chang KJ, Rigdon GC, Howard JL, McNutt RW . A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther 1993; 267: 852–7.
Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, et al. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80): a highly selective, nonpeptide delta opioid receptor agonist. J Med Chem 1994; 37: 2125–8.
Chang KJ, Porreca F, Woods JH, editors. The delta receptor. New York: Marcel Dekker; 2004.
Wild KD, McCormick J, Bilsky EJ, Vanderah T, McNutt RW, Chang KJ, et al. Antinociceptive actions of BW373U86 in the mouse. J Pharmacol Exp Ther 1993; 267: 858–65.
Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt RW, Chang KJ . A novel delta opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther 1993; 267: 875–82.
Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, et al. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther 1993; 267: 888–95.
Lee PH, McNutt RW, Chang KJ . A nonpeptidic delta opioid receptor agonist, BW373U86, attenuates the development and expression of morphine abstinence precipitated by naloxone in rat. J Pharmacol Exp Ther 1993; 267: 883–7.
Negus SS . Delta opioids and substance abuse. In: Chang KJ, Porreca F, Woods JH, editors. The delta receptor. New York: Marcel Dekker; 2004. p 401–30.
Hudzik TJ, Pietras MR, Caccese R, Bui KH, Yocca F, Paronis CA, et al. Effects of the delta opioid agonist AZD2327 upon operant behaviors and assessment of its potential for abuse. Pharmacol Biochem Behav 2014; 124: 48–57.
Jutkiewicz EM . The antidepressant-like effects of delta-opioid receptor agonists. Mol Interv 2006; 6: 162–9.
Mabrouk OS, Volta M, Marti M, Morari M . Stimulation of delta opioid receptors located in substantia nigra reticulata but not globus pallidus or striatum restores motor activity in 6-hydroxydopamine lesioned rats: new insights into the role of delta receptors in parkinsonism. J Neurochem 2008; 107: 1647–59.
Gross GJ, Fryer RM, Patel HH, Schultz JEJ . Cardioprotection and delta opioid receptors. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. p 451–67.
He X, Sandhu HK, Yang Y, Hua F, Belser N, Kim DH, Xia Y . Neuroprotection against hypoxia/ischemia: delta-opioid receptor-mediated cellular/molecular events. Cell Mol Life Sci 2013; 70: 2291–303.
Holt JD, Watson MJ, Chang JP, O'Neill SJ, Wei K, Pendergast W, et al. DPI-221 [4-((alpha-S)-alpha-((2S,5R)-2,5-dimethyl-4-(3-fluorobenzyl)-1-piperazinyl)benzyl)-N,N-diethylbenzamide]: a novel nonpeptide delta receptor agonist producing increased micturition interval in normal rats. J Pharmacol Exp Ther 2005; 315: 601–8.
O'Neill SJ, Collins MA, Pettit HO, McNutt RW, Chang KJ . Antagonistic modulation between the delta opioid agonist BW373U86 and the mu opioid agonist fentanyl in mice. J Pharmacol Exp Ther 1997; 282: 271–7.
Su YF, McNutt RW, Chang KJ . Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther 1998; 287: 815–23.
Pfeiffer A, Brantl V, Herz A, Emrich HM . Psychotomimesis mediated by kappa opiate receptors. Science 1986; 233: 774–6.
Pan ZZ . mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci 1998; 19: 94–8.
Wang YH, Sun JF, Tao YM, Chi ZQ, Liu JG . The role of kappa-opioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin 2010; 31: 1065–70.
Funada M, Suzuki T, Narita M, Misawa M, Nagase H . Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology 1993; 32: 1315–23.
Xi ZX, Fuller SA, Stein EA . Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther 1998; 284: 151–61.
Negus SS, Schrode K, Stevenson GW . Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 2008; 16: 386–99.
Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK . Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacol 2003; 28: 1125–39.
Wang YJ, Tao YM, Li FY, Wang YH, Xu XJ, Chen J, et al. Pharmacological characterization of ATPM [(−)-3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed kappa-agonist and mu-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behavior. J Pharmacol Exp Ther 2009; 329: 306–13.
Gengo PJ, Pettit HO, O'Neill SJ, Wei K, McNutt RW, Bishop MJ, et al. DPI-3290 [(+)-3-((alpha-R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)-N-(3-fluorophenyl)-N-methylbenzamide]. I. A mixed opioid agonist with potent antinociceptive activity. J Pharmacol Exp Ther 2003; 307: 1221–6.
Gengo PJ, Pettit HO, O'Neill SJ, Su YF, McNutt RW, Chang KJ . DPI-3290 [(+)-3-((alpha-R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)-N-(3-fluorophenyl)-N-methylbenzamide]. II. A mixed opioid agonist with potent antinociceptive activity and limited effects on respiratory function. J Pharmacol Exp Ther 2003; 307: 1227–33.
Gengo PJ, Chang KJ . Mixed opioid receptor agonists as a new class of agents for the treatment of moderate to severe pain. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. p 231–44.
Chang KJ, inventor; Ardent Pharmaceuticals, assignee. Enantiomerically pure opioid diarylmethylpiperazine and methods of using same. US Patent 6 924 288. 2005 Aug 2.
Chang KJ, Pendergast W, Biciunas KP, Wu ESC, inventors; Ardent Pharmaceuticals, assignee. Diarylmethylbenzylpiperazines and corresponding halobenzyl derivatives. US Patent 0 052 007. 2002 May 2.
Winger G, Woods JH . The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkeys. Drug Alcohol Depen 2001; 62: 181–9.
Winger G, Palmer R, Woods JH . Drug-reinforced responding: rapid determination of dose-response functions. Drug Alcohol Depen 1989; 24: 135–42.
Winger G, Skjoldager P, Woods JH . Effects of buprenorphine and other opioid agonists and antagonists on alfentanil- and cocaine-reinforced responding in rhesus monkeys. J Pharmacol Exp Ther 1992; 261: 311–7.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–54.
Cheng YC, Prusoff WH . Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973; 22: 3099–108.
Takagi H, Inukai T, Nakama M . A modification of Haffner's method for testing analgesics. Jpn J Pharmacol 1966; 16: 287–94.
Comer SD, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH . Discriminative stimulus effects of BW373U86: a nonpeptide ligand with selectivity for delta opioid receptors. J Pharmacol Exp Ther 1993; 267: 866–74.
Law PY . Delta opioid signaling and trafficking. In: Chang KJ, Porreca F, Woods JH, editors. The delta receptor. New York: Marcel Dekker; 2004. p 61–87.
Clark MJ, Traynor JR . Delta opioid receptors and G proteins. In: Chang KJ, Porreca F, Woods JH, editors. The Delta receptor. New York: Marcel Dekker; 2004. p 89–102.
Lacoste A, Evans CJ . Cloning of delta opioid receptor. In: Chang KJ, Porreca F, Woods JH, editors. The delta receptor. New York: Marcel Dekker; 2004. p 15–29.
Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, et al. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol 2000; 2: 25–9.
Feliciello A, Gottesman ME, Avvedimento EV . The biological functions of A-kinase anchor proteins. J Mol Biol 2001; 308: 99–114.
Almholt K, Tullin S, Skyggebjerg O, Scudder K, Thastrup O, Terry R . Changes in intracellular cAMP reported by a redistribution assay using a cAMP-dependent protein kinase-green fluorescent protein chimera. Cell Signal 2004; 16: 907–20.
Payza K . Binding and activity of opioid ligands at the cloned human delta, mu, and kappa receptors. In: Chang KJ, Porreca F, Woods JH, editors. The delta receptor. New York: Marcel Dekker; 2004. p 261–75.
Bianchi C, Franceschini J . Experimental observation on Haffner's method for testing analgesic drugs. Br J Pharmacol 1954; 9: 280–4.
Niemegeers CJE, Janssen PAJ . Alfentanil (R 39209), a particularly short-acting intravenous narcotic analgesic in rats. Drug Dev Res 1981; 1: 83–8.
Pattinson KT . Opioids and the control of respiration. Br J Anaesth 2008; 100: 747–58.
Koo CY, Eikermann M . Respiratory effects of opioids in perioperative medicine. Open Anaesthesiol J 2011; 5: 23–34.
George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 2000; 275: 26128–35.
Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA . Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci 2000; 20: RC110.
Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA . A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 2004; 101: 5135–9.
Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA . G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol 2011; 79: 1044–52.
Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry 2007; 46: 12997–3009.
Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S . Differential response to morphine of the oligomeric state of mu-opioid in the presence of delta-opioid receptors. Biochemistry 2011; 50: 2829–37.
Yekkirala AS, Kalyuzhny AE, Portoghese PS . Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [δ-Ala(2)-MePhe(4)-Glyol(5)]enkephalin as selective mu-delta agonists. ACS Chem Neurosci 2010; 1: 146–54.
Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS . Clinically employed opioid analgesics produce antinociception via mu-delta opioid receptor heteromers in Rhesus monkeys. ACS Chem Neurosci 2012; 3: 720–7.
Metcalf MD, Yekkirala AS, Powers MD, Kitto KF, Fairbanks CA, Wilcox GL, et al. The delta opioid receptor agonist SNC80 selectively activates heteromeric mu-delta opioid receptors. ACS Chem Neurosci 2012; 3: 505–9.
Acknowledgements
This study was financially supported by the Kunming University of Science and Technology (No 14078134), the Science and Technology Planning Project of Yunnan Province, China (No 2014FA002), the State Key Laboratory of Phytochemistry and Plant Resources in West China (No P2015-KF04), and the National Science and Technology Major Project of China (No 2012ZX09301003-001, 2012ZX09301003-003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available on the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary Information
Self-administration studies of DPI-125 (NIH11161) (DOC 1296 kb)
Rights and permissions
About this article
Cite this article
Yi, Sp., Kong, Qh., Li, Yl. et al. The opioid receptor triple agonist DPI-125 produces analgesia with less respiratory depression and reduced abuse liability. Acta Pharmacol Sin 38, 977–989 (2017). https://doi.org/10.1038/aps.2017.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.14