Abstract
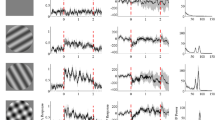
Spontaneous brain activity could affect processing if it were structured, . We show that neuron pairs in cat primary visual cortex exhibited correlated fluctuations in response latency, particularly when they had overlapping receptive fields or similar orientation preferences. Correlations occurred within and across hemispheres, but only when local field potentials (LFPs) oscillated in the gamma-frequency range (40–70 Hz). In this range, LFP fluctuations preceding response onset predicted response latencies; negative (positive) LFPs were associated with early (late) responses. Oscillations below 10 Hz caused covariations in response amplitude, but exhibited no columnar selectivity or coordinating effect on latencies. Thus, during high gamma activity, spontaneous activity exhibits distinct, column-specific correlation patterns. Consequently, cortical cells undergo coherent fluctuations in excitability that enhance temporal coherence of responses to contours that are spatially contiguous or have similar orientation. Because synchronized responses are more likely than dispersed responses to undergo rapid and joint processing, spontaneous activity may be important in early visual processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871 (1996).
Gawne, T. J., Kjaer, T. W. & Richmond, B. J. Latency: another potential code for feature binding in striate cortex. J. Neurophysiol. 76, 1356–1360 (1996).
Gur, M., Beylin, A. & Snodderly, D. M. Response variability of neurons in primary visual cortex (V1) of alert monkeys. J. Neurosci. 17, 2914–2920 (1997).
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998).
Alonso, J. M., Usrey, W. M. & Reid, R. C. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383, 815–819 (1996).
Brecht, M., Singer, W. & Engel, A. K. Correlation analysis of corticotectal interactions in the cat visual system. J. Neurophysiol. 79, 2394–2407 (1998).
Singer, W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49–65 (1999).
Tsodyks, M., Kenet, T., Grinvald, A. & Arieli, A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286, 1943–1946 (1999).
Lampl, I. & Yarom, Y. Subthreshold oscillations of the membrane potential: a functional synchronizing and timing device. J. Neurophysiol. 70, 2181–2186 (1993).
Volgushev, M., Chistiakova, M. & Singer, W. Modification of discharge patterns of neocortical neurons by induced oscillations of the membrane potential. Neuroscience 83, 15–25 (1998).
Nowak, L. G., Sanchez-Vives, M. V. & McCormick, D. A. Influence of low and high frequency inputs on spike timing in visual cortical neurons. Cereb. Cortex 7, 487–501 (1997).
Mainen, Z. F. & Sejnowski, T. J. Reliability of spike timing in neocortical neurons. Science 268, 1503–1506 (1995).
Stevens, C. F. & Zador, A. M. Input synchrony and the irregular firing of cortical neurons. Nat. Neurosci. 1, 210–217 (1998).
Azouz, R. & Gray, C. M. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J. Neurosci. 19, 2209–2223 (1999).
Lampl, I., Reichova, I. & Ferster, D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 22, 361–374 (1999).
Amzica, F. & Steriade, M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J. Neurophysiol. 73, 20–38 (1995).
Mitzdorf, U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 65, 37–100 (1985).
Engel, A. K., König, P., Kreiter, A. K. & Singer, W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252, 1177–1179 (1991).
Das, A. & Gilbert, C. D. Topography of contextual modulations mediated by short-range interactions in primary visual cortex. Nature 399, 655–661 (1999).
Schmidt, K. E., Kim, D. S., Singer, W., Bonhoeffer, T. & Löwel, S. Functional specificity of long-range intrinsic and interhemispheric connections in the visual cortex of strabismic cats. J. Neurosci. 17, 5480–5492 (1997).
Contreras, D. & Steriade, M. State-dependent fluctuations of low-frequency rhythms in corticothalamic networks. Neuroscience 76, 25–38 (1997).
Diesmann, M., Gewaltig, M. O. & Aertsen, A. Stable propagation of synchronous spiking in cortical neural networks. Nature 402, 529–533 (1999).
Thorpe, S., Fize, D. & Marlot, C. Speed of processing in the human visual system. Nature 381, 520–522 (1996).
Herculano-Houzel, S., Munk, M. H. J., Neuenschwander, S. & Singer, W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J. Neurosci. 19, 3992–4010 (1999).
Makeig, S. & Jung, T. P. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Brain Res. Cogn. Brain Res. 4, 15–25 (1996).
Bouyer, J. J., Montaron, M. F. & Rougeul, A. Fast fronto-parietal rhythms during combined focused attentive behaviour and immobility in cat: cortical and thalamic localizations. Electroencephalogr. Clin. Neurophysiol. 51, 244–252 (1981).
Sanes, J. N. & Donoghue, J. P. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc. Natl. Acad. Sci. USA 90, 4470–4474 (1993).
Murthy, V. N. & Fetz, E. E. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J. Neurophysiol. 76, 3949–3967 (1996).
Roelfsema, P. R., Engel, A. K., König, P. & Singer, W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 385, 157–161 (1997).
Fries, P., Reynolds, J. H., Rorie, A. E. & Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science (in press).
Swindale, N. V. Orientation tuning curves: empirical description and estimation of parameters. Biol. Cybern. 78, 45–56 (1998).
Acknowledgements
Supported by the MPG and the Heisenberg program of the DFG. We thank S. Herculano-Houzel for suggestions, J.-H. Schröder and M. Stephan for help with data analysis, and J. Reynolds and R. Desimone for help in recording monkey data and for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fries, P., Neuenschwander, S., Engel, A. et al. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci 4, 194–200 (2001). https://doi.org/10.1038/84032
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84032
This article is cited by
-
Sensitive period for rescuing parvalbumin interneurons connectivity and social behavior deficits caused by TSC1 loss
Nature Communications (2021)
-
Interacting Roles of COMT and GAD1 Genes in Patients with Treatment-Resistant Schizophrenia: a Genetic Association Study of Schizophrenia Patients and Healthy Controls
Journal of Molecular Neuroscience (2021)
-
Arche-writing and data-production in theory-oriented scientific practice: the case of free-viewing as experimental system to test the temporal correlation hypothesis
History and Philosophy of the Life Sciences (2021)
-
Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo
Nature Communications (2018)
-
Development of Intermodule Interactions in Field 18 in Kittens Reared in Different Visual Environments: Orientation Modules
Neuroscience and Behavioral Physiology (2018)