Abstract
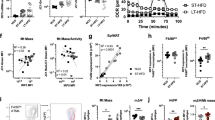
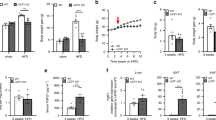
The gene Ucp2 is a member of a family of genes found in animals and plants, encoding a protein homologous to the brown fat uncoupling protein Ucp1 (refs 1–3). As Ucp2 is widely expressed in mammalian tissues4,5, uncouples respiration6 and resides within a region of genetic linkage to obesity4, a role in energy dissipation has been proposed. We demonstrate here, however, that mice lacking Ucp2 following targeted gene disruption are not obese and have a normal response to cold exposure or high-fat diet. Expression of Ucp2 is robust in spleen, lung and isolated macrophages4,5,7, suggesting a role for Ucp2 in immunity or inflammatory responsiveness4. We investigated the response to infection with Toxoplasma gondii in Ucp2−/− mice, and found that they are completely resistant to infection, in contrast with the lethality observed in wild-type littermates. Parasitic cysts and inflammation sites in brain were significantly reduced in Ucp2−/− mice (63% decrease, P<0.04). Macrophages from Ucp2 −/− mice generated more reactive oxygen species than wild-type mice (80% increase, P<0.001) in response to T. gondii, and had a fivefold greater toxoplasmacidal activity in vitro compared with wild-type mice (P<0.001 ), which was absent in the presence of a quencher of reactive oxygen species (ROS). Our results indicate a role for Ucp2 in the limitation of ROS and macrophage-mediated immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others

References
Ricquier, D. & Bouillaud, F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J. 345, 161–179 (2000).
Boss, O., Hagen, T. & Lowell, B.B. Uncoupling proteins 2 and 3. Potential regulators of mitochondrial energy metabolism. Diabetes 49, 143–156 (2000).
Laloi, M. et al. A plant cold-induced uncoupling protein. Nature 389, 135–136 (1997).
Fleury, C. et al. Uncoupling-protein-2: a novel gene linked to obesity and hyperinsulinemia . Nature Genet. 15, 269– 272 (1997).
Gimeno, R.E. et al. Cloning and characterization of an uncoupling protein homolog: a potential molecular mediator of human thermogenesis. Diabetes 46, 900–906 ( 1997).
Rial, E. et al. Retinoids activate proton transport by the uncoupling proteins UCP1 and UCP2. EMBO J. 21, 5827– 5833 (1999).
Larrouy, D. et al. Kupffer cells are a dominant site of uncoupling protein 2 expression in rat liver. Biochem. Biophys. Res. Commun. 235, 760–764 (1997).
Enerback, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90– 94 (1997).
Nègre-Salvayre, A. et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 11, 809–815 (1997).
Skulachev, V.P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta 1363, 100–124 (1998).
Nicholls, D.G. & Budd, S.L. Mitochondria and neuronal survival. Physiol. Rev. 80, 315 –360 (2000).
Arsenijevic, D., Girardier, L., Seydoux, J., Chang, H.R. & Dulloo, A.G. Altered energy balance and cytokine gene expression in a murine model of chronic infection with Toxoplasma gondii. Am. J. Physiol. 272, E908–917 (1997).
Richard, D. et al. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J. Comp. Neurol. 397, 549– 560 (1998).
Murray, H.W., Juangbhanich, C.W., Nathan, C.F. & Cohn, Z.A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J. Exp. Med. 150, 950– 964 (1979).
Marini, M., Frabetti, F., Zunica, G., Brandi, G. & Cantoni, O. Differential effect of L-histidine in human lymphocytes damaged by different oxygen radical producing systems . Mutat. Res. 301, 243– 248 (1993).
Zamora, R., Alaiz, M. & Hidalgo, F.J. Feed-back inhibition of oxidative stress by oxidized lipid/amino acid reaction products. Biochemistry 36 , 15765–15771 (1997).
Rothe, G. & Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin . J. Leukoc. Biol. 47, 440– 448 (1990).
Wallace, D.C. Mitochondrial diseases in man and mouse. Science 283 , 1482–1488 (1999).
Lee, F.Y.J. et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am. J. Physiol. Cell Physiol. 45, C386–C394 (1999).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000).
Vidal-Puig, A.J. et al. Energy metabolism in uncoupling protein 3 gene knockout mice . J. Biol. Chem. 275, 16258– 16266 (2000).
Diehl, A.M. & Hoek, J.B. Mitochondrial uncoupling: role of uncoupling protein anion carriers and relationship to thermogenesis and weight control “the benefits of losing control”. J. Bioenerg. Biomembr. 31, 493–506 (1999).
Cohen, B.A. Neurologic manifestations of toxoplasmosis in AIDS. Semin. Neurol. 19, 201–211 ( 1999).
Pecqueur, C. et al. Functional organization of the human uncoupling protein-2 gene, and juxtaposition to the uncoupling protein-3 gene. Biochem. Biophys. Res. Commun. 255, 40–46 (1999).
Surwit, R.S. et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44, 645–651 (1995).
Makioka, A. & Ohtomo, H. An increased DNA polymerase activity associated with virulence of Toxoplasma gondii. J. Parasitol. 81, 1021–1022 (1995).
Akinshina, G.T., Abakarova, E.G. & Kirillova, F.M. The relationship between the fate of the agent of toxoplasmosis, Toxoplasma gondii, in macrophages cultivated in vitro and the virulence of the parasites. Biull. Eksp. Biol. Med. 80, 60–63 (1975).
Rook, G.A., Steele, J., Umar, S. & Dockrell, H.M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J. Immunol. Methods 82, 161–167 ( 1985).
Acknowledgements
We thank C. Bock and the Duke University Transgenic Core for ES cell electroporation and blastocyst injections; L. Hale for immunohistochemistry of Ucp1 expression in adipose tissue sections; and W. Wetsel, B. Haynes, J.B. Weinberg, G. Sempowski and G. Taylor for discussions, reading of the manuscript and suggestions. This work was supported by Centre National de la Recherche Scientifique, Association de Recherches sur le Cancer, Institut de Recherches Servier, and Association Française contre les Myopathies (D. Ricquier), Université Laval (D. Richard), Human Frontier Science Program organization (grant RG-307/98 to D. Richard and D. Ricquier), NIH grants R01 DK54024 (S.C.). C.P. and E.C. were supported by doctoral fellowships of Association de Recherches sur le Cancer and Servier, respectively.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Arsenijevic, D., Onuma, H., Pecqueur, C. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26, 435–439 (2000). https://doi.org/10.1038/82565
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/82565
This article is cited by
-
Age-related hearing loss and its potential drug candidates: a systematic review
Chinese Medicine (2023)
-
Association between resting energy expenditure, diet and uncoupling protein 2 in obese women with normal and low resting energy expenditure
Nutrire (2023)
-
Sensing the oxygen and temperature in the adipose tissues – who’s sensing what?
Experimental & Molecular Medicine (2023)
-
Activation of UCP2 by anethole trithione suppresses neuroinflammation after intracerebral hemorrhage
Acta Pharmacologica Sinica (2022)
-
The cellular and functional complexity of thermogenic fat
Nature Reviews Molecular Cell Biology (2021)