Abstract
Small RNA molecules participate in a variety of activities in the cell: in a process known as RNA interference (RNAi), double-stranded RNA triggers the degradation of messenger RNA that has a matching sequence; the small RNA intermediates of this process can also modify gene expression in the nucleus1. Here we show that a single episode of RNAi in the nematode Caenorhabditis elegans can induce transcriptional silencing effects that are inherited indefinitely in the absence of the original trigger. Our findings may prove useful in the ongoing development of RNAi to treat disease.
Similar content being viewed by others
Main
It has been shown that phenotypes induced in C. elegans by RNAi can last for two or three generations2. Because the generation time of a worm is only three days, however, it is not clear whether this effect can be explained simply by a slow dilution of the silencing factors. We have therefore investigated the heritability of gene silencing by RNAi over many generations in C. elegans and used an RNAi screen to identify genes that may influence this inheritance. (For details of methods, see supplementary information.)
We injected wild-type Bristol N2 worms with a double-stranded RNA that targets the C. elegans gene ceh-13 for one generation. The Ceh-13 phenotype, in which the worm is small and dumpy, persisted in some animals indefinitely. Inheritance was not fully penetrant: only about 30% of the progeny of Ceh-13 worms inherited the phenotype. Wild-type siblings never had progeny with the Ceh-13 phenotype, and crossing worms that had a Ceh-13 phenotype with unaffected males showed that the trait is dominant. A single episode of RNAi can therefore induce heritable silencing that is not fully penetrant and behaves in a dominant fashion.
To show that this is a general phenomenon, we targeted 171 other genes by using a single treatment of RNAi and found 13 that could be inheritably silenced (among them were dpy-6, dpy-28 and unc-73; data not shown).
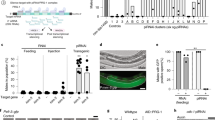
We also showed that a single transgenic copy of a gene (gfp) expressing green fluorescent protein (GFP) could be silenced, and the silencing inherited. We used animals expressing GFP under the control of a germline-specific promoter and created interference by feeding them bacteria that express double-stranded RNA homologous to gfp; progeny that did not express GFP were then transferred to new plates. In all siblings, GFP expression was reduced relative to wild-type expression (Fig. 1). We detected animals that had reduced GFP expression over 80 generations.
a, Four degrees of GFP expression in Caenorhabditis elegans are revealed using Nomarski optics (top panels) and ultraviolet illumination (bottom panels): ++, very bright, only observed in untreated transgenic NL3630 worms; −, +/− and +, weaker GFP signals from progeny of an RNAi-treated worm that did not express GFP. b, Worms were fed on bacteria expressing double-stranded RNA targeting the transgene gfp. Ten independent lines were quantified for 20 generations. Shown is the mean percentage of worms with the indicated amount of GFP expression in the progeny of a worm that did not express GFP.
Is RNAi the mechanism behind the initial silencing? There are two features of effective interference in C. elegans: it targets exons, not introns, and it depends on the canonical RNAi genes rde-1 and rde-4 (ref. 3) (see supplementary information). Tests for both show that RNAi is responsible for the effect, and this is further supported by our observation that genes are more likely to be indefinitely silenced in worms with a mutation in eri-1 (results not shown), which are hypersensitive to RNAi (ref. 4).
But RNAi probably does not underlie the inheritance mechanism — rde-1 and rde-4 are dispensable. To investigate further, we used a candidate RNAi screen to identify genes that affect the maintenance of silencing and found four that abolish inheritance when knocked out: hda-4 (a class II histone deacetylase), K03D10.3 (a histone acetyltransferase of the MYST family), isw-1 (a homologue of the yeast chromatin-remodelling ATPase ISW1) and mrg-1 (a chromo-domain protein) (see supplementary information).
The fact that these genes are all involved in chromatin remodelling suggests that the inheritance of RNAi-induced phenotypes is due to silencing at the transcriptional level. It may be that this is achieved by modification of specific residues in histone tails, because culturing worms in the presence of the histone deacetylase inhibitor trichostatin A relieves silencing.
Earlier work has revealed a link between RNAi and transcriptional silencing5 and inheritance of silencing for one generation in mice6. We have shown that RNAi can induce silencing effects that, once established, are inherited indefinitely over generations of sexual reproduction, in the absence of the trigger and of RNAi machinery.
References
Matzke, M. A. & Birchler, J. A. Nature Rev. Genet. 6, 24–35 (2005).
Grishok, A., Tabara, H. & Mello, C. C. Science 287, 2494–2497 (2000).
Tabara, H. et al. Cell 99, 123–132 (1999).
Kennedy, S., Wang, D. & Ruvkun, G. Nature 427, 645–649 (2004).
Lippman, Z. & Martienssen, R. Nature 431, 364–370 (2004).
Rassoulzadegan, M. et al. Nature 441, 469–474 (2006).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Methods
(DOC 70 kb)
Rights and permissions
About this article
Cite this article
Vastenhouw, N., Brunschwig, K., Okihara, K. et al. Long-term gene silencing by RNAi. Nature 442, 882 (2006). https://doi.org/10.1038/442882a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/442882a
This article is cited by
-
Tubular lysosome induction couples animal starvation to healthy aging
Nature Aging (2023)
-
Hypoxia-induced mitochondrial stress granules
Cell Death & Disease (2023)
-
C. elegans germ granules sculpt both germline and somatic RNAome
Nature Communications (2023)
-
Molecular mechanisms of transgenerational epigenetic inheritance
Nature Reviews Genetics (2022)
-
Reprogramming the piRNA pathway for multiplexed and transgenerational gene silencing in C. elegans
Nature Methods (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.