Abstract
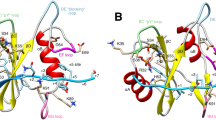
The protein Hck is a member of the Src family of non-receptor tyrosine kinases which is preferentially expressed in haematopoietic cells of the myeloid and B-lymphoid lineages1,2. Src kinases are inhibited by tyrosine-phosphorylation at a carboxy-terminal site3–9. The SH2 domains of these enzymes play an essential role in this regulation by binding to the tyrosine-phosphorylated tail8–11. The crystal structure of the downregulated form of Hck has been determined12 and reveals that the SH2 domain regulates enzymatic activity indirectly; intramolecular interactions between the SH3 and catalytic domains appear to stabilize an inactive form of the kinase. Here we compare the roles of the SH2 and SH3 domains in modulating the activity of Hck in an investigation of the C-terminally phosphorylated form of the enzyme. We show that addition of the HIV-1 Nef protein, which is a high-affinity ligand for the Hck SH3 domain, to either the downregulated or activated form of Hck causes a large increase in Hck catalytic activity. The intact SH3-binding motif in Nef is crucial for Hck activation. Our results indicate that binding of the Hck SH3 domain by Nef causes a more marked activation of the enzyme than does binding of the SH2 domain, suggesting a new mechanism for regulation of the activity of tyrosine kinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ziegler, S. F., Marth, J. C., Lewis, D. B. & Perlmutter, R. M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol. Cell. Biol. 7, 2276–2285 (1987).
Quintrell, N. et al. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol. Cell. Biol. 7, 2267–2275 (1987).
Cooper, J. A. & King, C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol. Cell. Biol. 6, 4467–4477 (1986).
Courtneidge, S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 4, 1471–1477 (1985).
Kmiecik, T. E. & Shalloway, D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49, 65–73 (1987).
Piwnica-Worms, H., Saunders, K. B., Roberts, T. M., Smith, A. E. & Cheng, S. H. Tyrosine phsophorylation regulates the biochemical and biological properties of pp60c-src. Cell 49, 75–82 (1987).
Cartwright, C. A., Eckhart, W., Simon, S. & Kaplan, P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell 49, 83–91 (1987).
Cooper, J. A. & Howell, B. The when and how of Src regulation. Cell 93, 1051–1054 (1993).
Superti-Furga, G. & Courtneidge, S. A. Structure-function relationships in Src family and related protein tyrosine kinases. BioEssays 17, 321–330 (1995).
Roussel, R. R., Brodeur, S. R., Shalloway, D. & Laudano, A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc. Natl Acad. Sci. USA 88, 10696–10700 (1991).
Liu, X. et al. Regulation of c-Src tyrosine kinase activity by the Sec SH2 domain. Oncogene 8, 1119–1126 (1993).
Sicheri, F., Moarefi, I. & Kuriyan, J. Crystal structure of the Src-family tyrosine kinase Hck. Nature 385, 602–609 (1997).
Cooper, J. A. & MacAuley, A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc. Natl Acad. Sci. USA 85, 4232–4236 (1988).
Barker, S. C. et al. characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry 34, 14843–14851 (1995).
Boerner, R. J. et al. Correlation of the phosphorylation states of pp60c-src with tyrosine kinase activity: the intramolecular pY530-SH2 complex retains significant activity if Y419 is phosphorylated. Biochemistry 35, 9519–9525 (1996).
Trono, D. HIV accessory proteins: leading roles for the supporting cast. Cell 82, 189–192 (1995).
Saksela, K., Cheng, G. & Baltimore, D. Proline-rich (PXXP) sequences in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14, 484–491 (1995).
Lee, C.-H. et al. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J 14, 5006–5015 (1995).
Pleiman, C. M., Hertz, W. M. & Cambier, J. C. Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science 263, 1609–1612 (1994).
Johnson, L. N., Noble, M. E. M. & Owen, D. J. Active and inactive protein kinases: structural basis for regulation. Cell 85, 149–158 (1996).
Alexandropoulos, K. & Baltimore, D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel, p130Cas-related protein, Sin. Genes Dev. 10, 1341–1355 (1996).
Till, J. H., Annan, R. S., Carr, S. A. & Miller, W. T. Use of synthetic peptide libraries and phosphopeptide-selective mass spectrometry to probe protein kinase substrate specificity. J. Biol. Chem. 269, 7423–7428 (1994).
Casnellie, J. H. Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Meth. Enzymol. 200, 115–120 (1991).
Feng, S., Chen, J. K., Yu, H., Simon, J. A. & Schreiber, S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science 266, 1241–1247 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moarefi, I., LaFevre-Bernt, M., Sicheri, F. et al. Activation of the Sire-family tyrosine kinase Hck by SH3 domain displacement. Nature 385, 650–653 (1997). https://doi.org/10.1038/385650a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/385650a0
This article is cited by
-
An allosteric switch between the activation loop and a c-terminal palindromic phospho-motif controls c-Src function
Nature Communications (2023)
-
Src signaling in a low-complexity unicellular kinome
Scientific Reports (2018)
-
Inhibition of N1-Src kinase by a specific SH3 peptide ligand reveals a role for N1-Src in neurite elongation by L1-CAM
Scientific Reports (2017)
-
A switch in nucleotide affinity governs activation of the Src and Tec family kinases
Scientific Reports (2017)
-
Auto-thiophosphorylation activity of Src tyrosine kinase
BMC Biochemistry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.