Abstract
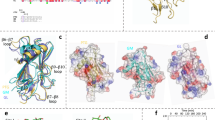
Phosphoglycerate kinase (PGK), a key enzyme in glycolysis, catalyses the transfer of a phosphoryl-group from 1,3-bis-phosphoglycerate to ADP to form 3-phosphoglycerate and ATP. Despite extensive kinetic and structural investigations over more than two decades, the conformation assumed by this enzyme during catalysis remained unknown. Here we present the 2.8 Å crystal structure of a ternary complex of PGK from Trypanosoma brucei, the causative agent of sleeping sickness. This structure determination relied on a procedure in which fragments containing less than 10% of the scattering mass were successively positioned in the unit cell to obtain phases. The PGK ternary complex exhibits a dramatic closing of the large cleft between the two domains seen in all previous studies1–6, thereby bringing the two ligands, 3-phosphoglycerate and ADP into close proximity. Our results demonstrate that PGK is a hinge-bending enzyme, reveal a novel mechanism in which substrate-induced effects combine synergistically to induce major conformational changes and, to our knowledge, afford the first observation of the PGK active site in a catalytic conformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blake, C. C. F. & Evans, P. R. J. Mol. Biol. 84, 585–601 (1974).
Watson, H. C. et al. EMBO J. 1, 1635–1640 (1982).
Harlos, K., Vas, M. & Blake, C. F. Proteins 12, 133–144 (1992).
Davies, G. J. et al. Acta Crystallogr. D 50, 202–209 (1994).
May, A., Vas, M., Harlos, K. & Blake, C. Proteins 24, 292–303 (1996).
McPhillips, T. M., Hsu, B. T., Sherman, M. A., Mas, M. T. & Rees, D. C. Biochemistry 35, 4118–4127 (1996).
Banks, R. D. et al. Nature 279, 773–777 (1979).
Mildvan, A. S. Phil. Trans. R. Soc. Lond. B 293, 65–74 (1981).
Knowles, J. R. Annu. Rev. Biochem. 49, 877–919 (1980).
Pappu, K. M., Gregory, J. D. & Serpersu, E. H. Arch. Biochem. Biophys. 311, 503–508 (1994).
Shirakihara, Y. & Evans, P. R. J. Mol. Biol. 204, 973–994 (1988).
Opperdoes, F. R. Annu. Rev. Microbiol. 41, 127–151 (1987).
Oh, B. Acta Crystallogr. D 51, 140–144 (1995).
Otwinowski, Z. in Data Collection and Processing (eds Sawyer, L., Isaacs, N. & Burley, S.) 56–62 (SERC Daresbury Laboratory, UK, 1993).
Matthews, B. W. J. Mol. Biol. 33, 491–497 (1968).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
CCP4 Acta Crystallogr. D 50, 760–763 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A. T. X-PLOR Version 3.1 (Yale Univ. Press, New Haven, CT, 1992).
Luthy, R., Bowie, J. U. & Eisenberg, D. Nature 356, 83–85 (1992).
Misset, O., Bos, O. J. M. & Opperdoes, F. R. Eur. J. Biochem. 157, 441–453 (1986).
Fothergill-Gilmore, L. A. & Michels, P. A. M. Progr. Biophys. Molec. Biol. 59, 105–235 (1993).
Kraulis, P. J. J. Appl. Crystallogr. 24, 946–950 (1991).
Scopes, R. K. Eur. J. Biochem. 85, 503–516 (1978).
Tompa, P., Hong, P. T. & Vas, M. Eur. J. Biochem. 154, 643–649 (1986).
Walker, P. A., Littlechild, J. A., Hall, L. & Watson, H. C. Eur. J. Biochem. 183, 49–55 (1989).
Graham, H. C. & Williams, R. J. P. Eur. J. Biochem. 197, 81–91 (1991).
Sherman, M. A., Fairbrother, W. J. & Mas, M. T. Prot. Sci. 1, 752–760 (1992).
Merritt, E. A. & Murphy, M. E. P. Acta Crystallogr. D 50, 869–873 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernstein, B., Michels, P. & Hol, W. Synergistic effects of substrate-induced conformational changes in phosphoglycerate kinase activation. Nature 385, 275–278 (1997). https://doi.org/10.1038/385275a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/385275a0
This article is cited by
-
Regulation of phosphoglycerate kinase 1 and its critical role in cancer
Cell Communication and Signaling (2023)
-
1H, 15N, 13C backbone resonance assignments of human phosphoglycerate kinase in a transition state analogue complex with ADP, 3-phosphoglycerate and magnesium trifluoride
Biomolecular NMR Assignments (2017)
-
The dynamics of single protein molecules is non-equilibrium and self-similar over thirteen decades in time
Nature Physics (2016)
-
Terazosin activates Pgk1 and Hsp90 to promote stress resistance
Nature Chemical Biology (2015)
-
Drug targets in Leishmania
Journal of Parasitic Diseases (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.