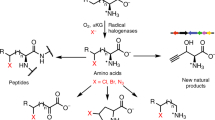
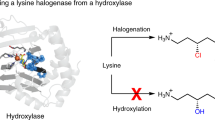
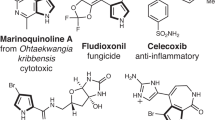
Abstract
Crystal structures of haloalkane dehalogenase were determined in the presence of the substrate 1,2-dichloroethane. At pH 5 and 4 °C, substrate is bound in the active site without being converted; warming to room temperature causes the substrate's carbon–chlorine bond to be broken, producing a chloride ion with concomitant alkylation of the active-site residue Asp124. At pH 6 and room temperature the alkylated enzyme is hydrolysed by a water molecule activated by the His289–Asp260 pair in the active site. These results show that catalysis by the dehalogenase proceeds by a two-step mechanism involving an ester intermediate covalently bound at Asp124.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Janssen, D. B., Scheper, A., Dijkhuizen, L. & Witholt, B. Appl. envir. Microbiol. 49, 673–677 (1985).
Keuning, S., Janssen, D. B. & Witholt, B. J. Bact. 163, 635–639 (1985).
Janssen, D. B. et al. J. Bact. 171, 6791–6799 (1989).
Franken, S. M., Rozeboom, H. J., Kalk, K. H. & Dijkstra, B. W. EMBO J. 10, 1297–1302 (1991).
Ollis, D. L. et al. Protein Engng 5, 197–211 (1992).
Sussman, J. L. et al. Science 253, 872–879 (1991).
Pathak, D. & Ollis, D. J. Molec. Biol. 214, 497–525 (1990).
Liao, D.-I. & Remington, S. J. J. biol. Chem. 265, 6528–6531 (1990).
Liao, D.-I., Breddam, K., Sweet, R. M., Bullock, T. & Remington, S. J. Biochemistry 31, 9796–9812 (1992).
Schrag, J. D., Li, Y., Wu, S. & Cygler, M. Nature 351, 761–764 (1991).
Janssen, D. B. et al. Eur. J. Biochem. 171, 67–72 (1988).
Rozeboom, H. J., Kingma, J., Janssen, D. B. & Dijkstra, B. W. J. molec. Biol. 200, 611–612 (1988).
Froede, H. C. & Wilson, I. B. J. biol. Chem. 259, 11010–11013 (1984).
Douglas, K. T., Nakagawa, Y. & Kaiser, E. T. J. Am. chem. Soc. 98, 8231–8236 (1976).
Hol, W. G. J., van Duijnen, P. T. & Berendsen, H. J. C. Nature 273, 443–446 (1978).
Hol, W. G. J. Prog. Biophys. molec.. Biol. 45, 149–195 (1985).
Kraut, J. A. Rev. Biochem. 46, 331–358 (1977).
McPhalen, C. A. & James, M. N. G. Biochemistry 27, 6582–6598 (1988).
Hajdu, J. et al. Nature 329, 178–181 (1987).
Singer, P. T., Smalas, A., Carty, R. P., Mangel, W. F. & Sweet, R. M. Science 259, 669–673 (1993).
Strynadka, N. C. J. et al. Nature 359, 700–705 (1992).
Messerschmidt, A. & Pflugrath, J. W. J. appl. Crystallogr. 20, 306–315 (1987).
Kabsch, W. J. appl. Crystallogr. 21, 916–924 (1988).
Hamilton, W. C., Rollet, J. S. & Sparks, R. A. Acta crystaltogr. 18, 129–130 (1965).
Read, R. Acta crystallogr. A42, 140–149 (1986).
Tronrud, D. E., ten Eyk, L. F. & Matthews, B. W. Acta crystallogr. A43, 489–501 (1987).
Verschueren, K. H. G., Franken, S. M., Rozeboom, H. J., Kalk, K. H. & Dijkstra, B. W. J. molec. Biol. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verschueren, K., Seljée, F., Rozeboom, H. et al. Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 363, 693–698 (1993). https://doi.org/10.1038/363693a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/363693a0
This article is cited by
-
Catalytic mechanism for Renilla-type luciferases
Nature Catalysis (2023)
-
Molecular Docking and Site-Directed Mutagenesis of Dichloromethane Dehalogenase to Improve Enzyme Activity for Dichloromethane Degradation
Applied Biochemistry and Biotechnology (2020)
-
Enzymatic defluorination of fluorinated compounds
Applied Biological Chemistry (2019)
-
In silico design of potentially functional artificial metallo-haloalkane dehalogenase containing catalytic zinc
3 Biotech (2018)
-
The complete genome sequence of the cold adapted crude-oil degrader: Pedobacter steynii DX4
Standards in Genomic Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.