Abstract
The checkpoint kinase proteins Mec1 and Rad53 are required in the budding yeast, Saccharomyces cerevisiae, to maintain cell viability in the presence of drugs causing damage to DNA or arrest of DNA replication forks1,2,3. It is thought that they act by inhibiting cell cycle progression, allowing time for DNA repair to take place. Mec1 and Rad53 also slow S phase progression in response to DNA alkylation4, although the mechanism for this and its relative importance in protecting cells from DNA damage have not been determined . Here we show that the DNA-alkylating agent methyl methanesulphonate (MMS) profoundly reduces the rate of DNA replication fork progression; however, this moderation does not require Rad53 or Mec1. The accelerated S phase in checkpoint mutants4, therefore, is primarily a consequence of inappropriate initiation events5,6,7. Wild-type cells ultimately complete DNA replication in the presence of MMS. In contrast, replication forks in checkpoint mutants collapse irreversibly at high rates. Moreover, the cytotoxicity of MMS in checkpoint mutants occurs specifically when cells are allowed to enter S phase with DNA damage. Thus, preventing damage-induced DNA replication fork catastrophe seems to be a primary mechanism by which checkpoints preserve viability in the face of DNA alkylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lowndes, N. F. & Murguia, J. R. Sensing and responding to DNA damage. Curr. Opin. Genet. Dev. 10, 17–25 (2000).
Zhou, B. B. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Rhind, N. & Russell, P. Checkpoints: it takes more than time to heal some wounds. Curr. Biol. 10, R908–R911 (2000).
Paulovich, A. G. & Hartwell, L. H. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82, 841–847 (1995).
Shirahige, K. et al. Regulation of DNA-replication origins during cell-cycle progression. Nature 395, 618–621 (1998).
Santocanale, C. & Diffley, J. F. X. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615–618 (1998).
Santocanale, C., Sharma, K. & Diffley, J. F. X. Activation of dormant origins of DNA replication in budding yeast. Genes Dev. 13, 2360–2364 (1999).
Reynolds, A. E., McCarroll, R. M., Newlon, C. S. & Fangman, W. L. Time of replication of ARS elements along yeast chromosome III. Mol. Cell. Biol. 9, 4488–4494 (1989).
Tercero, J. A., Labib, K. & Diffley, J. F. X. DNA synthesis at individual replication forks requires the essential initiation factor, Cdc45p. EMBO J. 19, 2082–2093 (2000).
Yamashita, M. et al. The efficiency and timing of initiation of replication of multiple replicons of Saccharomyces cerevisiae chromosome VI. Genes Cells 2, 655–665 (1997).
Friedman, K. L., Brewer, B. J. & Fangman, W. L. Replication profile of Saccharomyces cerevisiae chromosome VI. Genes Cells 2, 667–678 (1997).
Chan, C. S. & Tye, B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell 33, 563–573 (1983).
Craven, R. J. & Petes, T. D. Involvement of the checkpoint protein Mec1p in silencing of gene expression at telomeres in Saccharomyces cerevisiae. Mol. Cell. Biol. 20, 2378–2384 (2000).
Longhese, M. P., Paciotti, V., Neecke, H. & Lucchini, G. Checkpoint proteins influence telomeric silencing and length maintenance in budding yeast. Genetics 155, 1577–1591 (2000).
Rivin, C. J. & Fangman, W. L. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J. Cell Biol. 85, 108–115 (1980).
Shirahige, K., Iwasaki, T., Rashid, M. B., Ogasawara, N. & Yoshikawa, H. Location and characterization of autonomously replicating sequences from chromosome VI of Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 5043–5056 (1993).
Stevenson, J. B. & Gottschling, D. E. Telomeric chromatin modulates replication timing near chromosome ends. Genes Dev. 13, 146–151 (1999).
Campbell, J. L. & Newlon, C. S. in The Molecular Biology of the Yeast Saccharomyces; Genome Dynamics, Protein Synthesis and Energetics (eds Broach, J. R., Pringle, J. R. & Jones, E. W.) 41–146 (Cold Spring Harbor Press, Cold Spring Harbor, 1991).
Higgins, N. P., Kato, K. & Strauss, B. A model for replication repair in mammalian cells. J. Mol. Biol. 101, 417–425 (1976).
Schwartz, J. L. Monofunctional alkylating agent-induced S-phase-dependent DNA damage. Mutat. Res. 216, 111–118 (1989).
Dimitrova, D. S. & Gilbert, D. M. Temporally coordinated assembly and disassembly of replication factories in the absence of DNA synthesis. Nature Cell Biol. 2, 686–694 (2000).
Zhao, X., Muller, E. G. & Rothstein, R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2, 329–340 (1998).
Acknowledgements
We thank past and present members of the laboratory for discussions and K. Labib and D. G. Quintana for critical reading of the manuscript. We also thank M. Foiani and colleagues for discussions and for communicating unpublished results. This work was supported by the Imperial Cancer Research Fund. J.A.T. was supported by a Human Frontier Science Program fellowship.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figures

Figure 1
(GIF 29.5 KB)
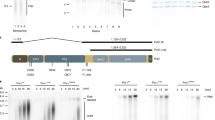
Effect of MMS on viability and S phase progression. Cells were arrested in G1 with a factor and released into fresh medium containing 0.033% MMS at 30oC as in ref1. Viability (a) and S phase progression (b) were determined. In (c) the density substitution approach used in subsequent experiments is diagrammed. DNA substituted with heavy isotopes is shown as thick black lines while DNA made in the presence of light isotopes is shown as thinner grey lines. Vertical lines represent restriction sites and the positions of probes for DNA blot hybridisation are shown. The FACS profile in (b) was taken from the density substitution experiments described in figures 1 and 2 (main text).

Figure 2
(GIF 40.4 KB)
Replication fork progression in MMS in a checkpoint-proficient strain. Replication in a MEC1+, RAD53+, SML1+ strain (YJT80) with MMS was followed exactly as in Fig.1 (main text).

Figure 3
(GIF 36.4 KB)
Replication Fork Progression in the Absence of MMS. Replication in the indicated strains was examined as in Fig. 1 (main text) in a factor and after one hour release from a factor in the absence of MMS.

Figure 4
(GIF 10.4 KB)
Fork rate and fork collapse rate determination for checkpoint-proficient and -deficient strains. Fork rate was determined by plotting the fraction of each restriction fragment replicated against time. The point at which 50% of a fragment has replicated is termed ‘TREP’. In Supplementary Figure 4a we have plotted TREP against distance of the midpoint of each restriction fragment across the entire region. The slope of this line provides the replication fork rate. In (a), TREP is plotted against distance from the ARS consensus sequence for wild type (m) and sml1∆ (l) strains. The overall fork rate across this region is approximately 285 bp/min in wild type cells and 301 bp/min in the sml1∆ cells. These rates are 5-10 times slower than average fork rates in the absence of MMS. Forks slow down toward the end of the experiment; fork rate across the first three fragments in sml1∆ cells is 475 bp/min and 233 bp/min over the last three fragments. This may be due to accumulated damage due to continued exposure to MMS or to normal slowing of replication forks, a phenomenon previously documented for the ends of chromosome III. (b) Because of complications caused by secondary origin activation in the checkpoint mutants, fork rates could not be determined across fragments 4-6. However, fork rates across the first 20 kb (fragments 1-3) could be determined as described above. TREP was plotted against distance over the first 20 kb (fragments 1-3) for the sml1∆rad53∆ (o) and sml1∆mec1∆ (n) strains. Lines were determined by linear regression. From this data we calculate that forks move at 256 bp/min in the sml1∆ mec1∆ strain and 318 bp/min in the sml1∆ rad53∆ strain. These rates are actually slower than the rates of fork progression across the same 20 kb region in the checkpoint proficient strains (see above). We note that the average replicon size in yeast is approximately 40 kb, so the average replication fork travels ~20 kb. Thus, fork rate in this experiment has been determined over a similar distance and is likely to be representative of the average replicon. (d) Replication fork breakdown rates. The percent terminally unreplicated DNA at the end of the experiment (Fig. 2, main text) for fragments 1-3 was plotted against distance from the ACS for the sml1∆rad53∆ (o) and sml1∆mec1∆ (n) strains. Fork breakdown rates were determined as follows. Because of the near-linear accumulation of terminally unreplicated DNA across fragments 1-3, we assume that forks break down at approximately random positions. While this is almost certainly an oversimplification and DNA sequence will likely play a role in breakdown rates, it is a helpful assumption for the following calculations. The slope of the line, after linear regression, in Supplementary Figure 4c can be used to determine the fraction of terminally unreplicated DNA at any position. At 20 kb, this number is 38.7% in the rad53 mutant and 46% in the mec1 mutant. Conversely, 61.3% (rad53) and 54% (mec1) of the forks have not broken down before 20 kb. The probability, p, that a fork will not break down at any single base pair position, then, is given by the formula pn=0.613 (rad53) or 0.54 (mec1) where n is the total number of base pairs (20,000). From this p=0.999969191 (rad53) and 0.999975531 (mec1). This probability, with the formula above, can then be used to calculate the fraction of forks which will have broken down before reaching any position along the replicon. Using this, we calculate that forks terminate irreversibly at approximately the same rate in the two mutants (2.4% over 1 kb, rad53; 3.0% over 1 kb, mec1).
Table
References for Supplementary Information
-
1.
Paulovich, A. G. & Hartwell, L. H. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82, 841-847 (1995).
-
2.
Reynolds, A. E., McCarroll, R. M., Newlon, C. S. & Fangman, W. L. Time of replication of ARS elements along yeast chromosome III. Mol Cell Biol 9, 4488-4494 (1989).
-
3.
Rivin, C. J. & Fangman, W. L. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J Cell Biol 85, 108-115 (1980).
-
4.
Sikorski, R. S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27 (1989).
-
5.
Tercero, J. A., Labib, K. & Diffley, J. F. X. DNA synthesis at individual replication forks requires the essential initiation factor, Cdc45p. EMBO J 19, 2082-2093 (2000).
Rights and permissions
About this article
Cite this article
Tercero, J., Diffley, J. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412, 553–557 (2001). https://doi.org/10.1038/35087607
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35087607
This article is cited by
-
ATR kinase supports normal proliferation in the early S phase by preventing replication resource exhaustion
Nature Communications (2023)
-
Cell cycle control in cancer
Nature Reviews Molecular Cell Biology (2022)
-
The budding yeast protein Chl1p is required for delaying progression through G1/S phase after DNA damage
Cell Division (2021)
-
Rad53 limits CMG helicase uncoupling from DNA synthesis at replication forks
Nature Structural & Molecular Biology (2020)
-
A Novel Cell-Penetrating Antibody Fragment Inhibits the DNA Repair Protein RAD51
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.