Abstract
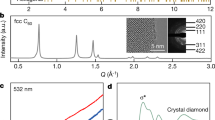
Synthetic diamond is formed commercially using high-pressure1, chemical-vapour-deposition2 and shock-wave3 processes, but these approaches have serious limitations owing to low production volumes and high costs. Recently suggested alternative methods of diamond growth include plasma activation4, high pressures5, exotic precursors6,7 or explosive mixtures8, but they suffer from very low yield and are intrinsically limited to small volumes or thin films. Here we report the synthesis of nano- and micro-crystalline diamond-structured carbon, with cubic and hexagonal structure, by extracting silicon from silicon carbide in chlorine-containing gases at ambient pressure and temperatures not exceeding 1,000 °C. The presence of hydrogen in the gas mixture leads to a stable conversion of silicon carbide to diamond-structured carbon with an average crystallite size ranging from 5 to 10 nanometres. The linear reaction kinetics allows transformation to any depth, so that the whole silicon carbide sample can be converted to carbon. Nanocrystalline coatings of diamond-structured carbon produced by this route show promising mechanical properties, with hardness values in excess of 50 GPa and Young's moduli up to 800 GPa. Our approach should be applicable to large-scale production of crystalline diamond-structured carbon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bundy, F. P., Hall, H. T., Strong, H. M. & Wentorf, R. H. Man-made diamonds. Nature 176, 51 (1955).
Angus, J. C. & Hayman, C. C. Low-pressure, metastable growth of diamond and “diamondlike” phases. Science 241, 913–921 (1988).
Burkhard, G., Dan, K., Tanabe, Y., Sawaoka, A. B. & Yamada, K. Carbon phase transition by dynamic shock compression of a copper/graphite powder mixture. Jpn J. Appl. Phys. 33, L876–L879 (1994).
Roy, R. et al. New process for 1 atm diamond synthesis: from metallic solutions. Innov. Mater. Res. 1, 65–87 (1996).
Zhao, X.-Z., Roy, R., Cherian, K. A. & Badzian, A. Hydrothermal growth of diamond in metal-C-H2O systems. Nature 385, 513–515 (1997).
Gruen, D. M., Liu, S., Krauss, A. R. & Pan, X. Buckyball microwave plasmas: fragmentation and diamond-film growth. J. Appl. Phys. 75, 1758–1763 (1994).
Regueiro, M. N., Monceau, P. & Hodeau, J.-L. Crushing C60 to diamond at room temperature. Nature 355, 237–239 (1992).
Li, Y. et al. A reduction-pyrolysis-catalysis synthesis of diamond. Science 281, 246–247 (1998).
Gogotsi, Y. G. & Yoshimura, M. Formation of carbon films on carbides under hydrothermal conditions. Nature 367, 628–630 (1994).
Gogotsi, Y. G., Kofstad, P., Nickel, K. G. & Yoshimura, M. Formation of sp3-bonded carbon upon hydrothermal treatment of SiC. Diamond Relat. Mater. 5, 151–162 (1996).
Gogotsi, Y. G., Jeon, J. D. & McNallan, M. J. Carbon coatings on silicon carbide by reaction with chlorine-containing gases. J. Mater. Chem. 7, 1841–1848 (1997).
Gogotsi, Y. in Proc. NATO ARW on Nanostructured Films and Coatings (eds Chow, G.-M., Ovid’ko, I. A. & Tsakalakos, T.) 25–40 (Kluwer, Dordrecht, 1999).
Gogotsi, Y. G., Nickel, K. G. & Kofstad, P. Hydrothermal synthesis of diamond from diamond-seeded β-SiC powder. J. Mater. Chem. 5, 2313–2314 (1995).
Silva, S. R. P., Amaratunga, G. A. J., Salje, E. K. H. & Knowles, K. H. Evidence of hexagonal diamond in plasma-deposited carbon films. J. Mater. Sci. 29, 4962–4963 (1994).
Zarrabian, M., Fourches-Coulon, N., Turban, G., Marhic, C. & Lancin, M. Observation of nanocrystalline diamond in diamondlike carbon films deposited at room temperature in electron cyclotron resonance plasma. Appl. Phys. Lett. 70, 253–255 (1997).
Hough, R. M. et al. Diamond and silicon carbide in impact melt rock from the Ries impact crater. Nature 378, 41–44 (1995).
Rossi, M., Vitali, G., Terranova, M. L. & Sessa, V. Experimental evidence of different crystalline forms in chemical vapour deposited diamond films. Appl. Phys. Lett. 63, 2765–2767 (1993).
Kofstad, P. High Temperature Corrosion (Elsevier, London, 1988).
Gruen, D. M. Nanocrystalline diamond films. Annu. Rev. Mater. Sci. 29, 211–259 (1999).
Grannen, K. J. & Chang, R. P. H. Diamond growth on carbide surfaces using a selective etching technique. J. Mater. Res. 9, 2154–2163 (1994).
Lannon, J. M. J., Gold, J. S. & Stinespring, C. D. Hydrogen ion interactions with silicon carbide and the nucleation of diamond thin films. J. Appl. Phys. 77, 3823–3830 (1995).
Heera, V., Skorupa, W., Pecz, B. & Dobos, L. Ion beam synthesis of graphite and diamond in silicon carbide. Appl. Phys. Lett. 76, 2847–2849 (2000).
Spitsyn, B. V. in Handbook of Crystal Growth (ed. Hurle, D. T. J.) 401–456 (Elsevier, London, 1994).
Gogotsi, Y. et al. in Proc. NATO ASI on Functional Gradient Materials and Surface Layers Prepared by Fine Particles Technology (eds Baraton, M.-I. & Uvarova, I. V.) 239–255 (Kluwer, Dordrecht, 2001).
Wang, J.-T., Cao, C.-B. & Zheng, P.-J. Theoretical aspects of low pressure diamond synthesis. J. Electrochem. Soc. 141, 278–281 (1994).
Leung, I., Guo, W., Friedman, I. & Gleason, J. Natural occurrence of silicon carbide in a diamondiferous kimberlite from Fuxian. Nature 346, 352–354 (1990).
Daulton, T. L. & Ozima, M. Radiation-induced diamond formation in uranium-rich carbonaceous materials. Science 271, 1260–1262 (1996).
Gogotsi, Y. et al. Formation of carbon coatings on SiC fibres by selective etching in halogens and supercritical water. Ceram. Eng. Sci. Proc. 19, 87–94 (1998).
Kyutt, R. N., Smorgonskaya, E. A., Danishevskii, A. M., Gordeev, S. K. & Grechinskaya, A. V. Structural study of nanoporous carbon produced from polycrystalline carbide materials: small angle X-ray scattering. Phys. Solid State 41, 1359–1363 (1999).
Zheng, J., Ekström, T. C., Gordeev, S. K. & Jacob, M. Carbon with an onion-like structure obtained by chlorinating titanium carbide. J. Mater. Chem. 10, 1039–1041 (2000).
Ersoy, D. A., McNallan, M. J., Gogotsi, Y. & Erdemir, A. Tribological properties of carbon coatings produced by high temperature chlorination of silicon carbide. STLE Tribol. Trans. 43, 809–815 (2000).
Hirai, H. & Kondo, K.-I. Modified phases of diamond formed under shock compression and rapid quenching. Science 253, 772–774 (1991).
Acknowledgements
We thank A. Nicholls for discussions and help with TEM analysis. The work done at UIC was supported by the US NSF, and the work done at Drexel University was supported by DARPA via ONR contract. The electron microscopes used in this work are operated by the Research Resources Center at UIC. The JEM-2010F purchase was supported by the NSF.
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Figure 1.
(JPG 12.4 KB)
Convergent-beam electron diffraction (CBED) patterns fromnanocrystals (5-10 nm size) in a sample treated in Ar-3.5% chlorine at1000ºC. The diamond-containing area was within a micrometer from theSiC/carbon interface. a, b, may be attributed to cubic Fd3m or F43mstructures with reflections at 0.206 nm (111) and 0.126 nm (022), as well asforbidden diamond reflections. c,d, may be attributed to hexagonal diamond(lonsdaleite) with reflections at 0.219 (100) and 0.126 nm (110). e, EDSspectrum showing that the analyzed material is nearly pure carbon. Tracesof amorphous silica were present due to oxygen impurity in the gas. Thecopper peak comes from the supporting grid. Other EDS spectra from theanalyzed areas showed even lower content of impurities in carbon.

Figure 2.
(JPG 25.2 KB)
SAD pattern from the nanocrystalline film. Sharp Braggreflections are visible up to the order of (800), indicating good crystallinity.No scattering intensity from either graphite or amorphous carbon can beseen, suggesting that the film is pure diamond, but high intensity of forbiddenreflections suggests a lower symmetry (F&4macr;3m) or impurity superstructure.Sample was sintered α-SiC treated in 2.77% CI2-1.04%H2 (balance Ar) for 5hours at 1000ºC.
Rights and permissions
About this article
Cite this article
Gogotsi, Y., Welz, S., Ersoy, D. et al. Conversion of silicon carbide to crystalline diamond-structured carbon at ambient pressure. Nature 411, 283–287 (2001). https://doi.org/10.1038/35077031
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35077031
This article is cited by
-
Effect of catalyst type on the type and size of nano-diamond synthesized by chlorine gas etching of Ti3C2 MXene under ambient pressure
Journal of Materials Science (2024)
-
Thermodynamic aspects of co-formation of different condensed phases from CH4–WCl6 gas mixtures diluted in H2
Applied Physics A (2018)
-
RETRACTED ARTICLE: Fundamental Discovery of New Phases and Direct Conversion of Carbon into Diamond and hBN into cBN and Properties
Metallurgical and Materials Transactions A (2016)
-
Surprising synthesis of nanodiamond from single-walled carbon nanotubes by the spark plasma sintering process
Electronic Materials Letters (2016)
-
Graphene from Amorphous Titanium Carbide by Chlorination under 200°C and Atmospheric Pressures
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.