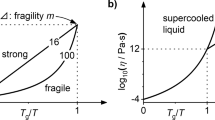
Abstract
Although liquids normally crystallize on cooling, there are members of all liquid types (including molecular, ionic and metallic) that supercool and then solidify at their glass transition temperature, Tg. This continuous solidification process exhibits great diversity within each class of liquid—both in the steepness of the viscosity–temperature profile, and in the rate at which the excess entropy of the liquid over the crystalline phase changes as Tg is approached. However, the source of the diversity is unknown. The viscosity and associated relaxation time behaviour have been classified between ‘strong’ and ‘fragile’ extremes, using Tg as a scaling parameter1, but attempts to correlate such kinetic properties with the thermodynamic behaviour have been controversial2,3. Here we show that the kinetic fragility can be correlated with a scaled quantity representing excess entropy, using data over the entire fragility range and embracing liquids of all classes. The excess entropy used in our correlation contains both configurational and vibration-related contributions. In order to reconcile our correlation with existing theory and simulations, we propose that variations in the fragility of liquids originate in differences between their vibrational heat capacities, harmonic and anharmonic, which we interpret in terms of an energy landscape. The differences evidently relate to behaviour of low-energy modes near and below the boson peak.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Angell, C. A. Relaxation in liquids, polymers and plastic crystals - strong/fragile patterns and problems. J. Non-Cryst. Solids 131-133, 13–31 (1991).
Ito, K., Moynihan, C. T. & Angell, C. A. Thermodynamic determination of fragility in liquids and a fragile-to-strong liquid transition in water. Nature 398, 492–495 (1999).
Ngai, K. L. & Yamamuro, O. Thermodynamic fragility and kinetic fragility in supercooled liquids: a missing link. J. Chem. Phys. 111, 10403–10406 (1999).
Sastry, S., Debenedetti, P. G. & Stillinger, F. H. Signatures of distinct dynamical regimes in the energy landscape of a glassforming liquid. Nature 393, 554–557 (1998).
Speedy, R. J. Relations between a liquid and its glasses. J. Phys. Chem. B 103, 4060–4065 (1999).
Roland, C. M., Santangelo, P. G. & Ngai, K. L. The application of the energy landscape model to polymers. J. Chem. Phys. 111, 5593–5598 (1999).
Speedy, R. J. The hard sphere glass transition. Mol. Phys. 95, 169–178 (1998).
Sciortino, F., Kob, W. & Tartaglia, P. Inherent structure entropy of supercooled liquids. Phys. Rev. Lett. 83, 3214–3217 (1999).
Buechner, S. & Heuer, A. The potential energy landscape of a model glassformer: thermodynamics, anharmonicities, and finite size effects. Phys. Rev. E 60, 6507–6518 (1999).
Coluzzi, B., Verrocchio, P., Mezard, M. & Parisi, G. Lennard-Jones binary mixture: a thermodynamical approach to glass transition. J. Chem. Phys. 112, 2933–2944 (2000).
Scala, A., Starr, F., La Nave, E., Sciortino, F. & Stanley, H. E. Configurational entropy and diffusion of supercooled water. Nature 406, 166–169 (2000).
Sastry, S. Liquid limits: the glass transition and liquid-gas spinodal boundaries of metastable liquids. Phys. Rev. Lett. 85, 590–593 (2000).
Sastry, S. The relationship between fragility, configurational entropy and the potential energy landscape of glassforming liquids. Nature 409, 164–167 (2001).
Richet, P. Viscosity and configurational entropy of silicate melts. Geochim. Cosmochim. Acta 48, 471–483 (1984).
Richet, P. & Bottinga, Y. Glass transitions and thermodynamic properties of amorphous SiO2, NaAlSinO2n+2 and KAlSi3O8. Geochim. Cosmochim. Acta 48, 453–470 (1984).
Adam, G. & Gibbs, J. H. On the temperature dependence of cooperative relaxation properties in glassforming liquids. J. Chem. Phys. 43, 139–146 (1965).
Angell, C. A. Entropy and fragility in supercooled liquids. J. Res. NIST 102, 171–185 (1997).
Richert, R. & Angell, C. A. Dynamics of glassforming liquids. IV: On the link between molecular dynamics and configurational entropy. J. Chem. Phys. 108, 9016–9026 (1998).
Goldstein, M. Viscous liquids and the glass transition: a potential energy barrier picture. J. Chem. Phys. 51, 3728–3729 (1969).
Stillinger, F. H. & Weber, T. A. Packing structures and transitions in liquids and solids. Science 225, 983–989 (1984).
Goldstein, M. Viscous liquids and the glass transition: sources of the excess heat capacity. J. Chem. Phys. 64, 4767–4773 (1976).
Johari, G. P. Contributions to the entropy of a glass and liquid, and the dielectric relaxation time. J. Chem. Phys. 112, 7518–7523 (2000).
Greet, R. J. & Turnbull, D. Test of Adam-Gibbs liquid viscosity model with o-terphenyl specific-heat data. J. Chem. Phys. 47, 2185–2190 (1967).
Magill, J. H. Physical properties of aromatic hydrocarbons. III. A test of the Adam-Gibbs relaxation model for glass formers based on the heat-capacity data of 1,3,5-tri-α-naphthylbenzene. J. Chem. Phys. 47, 2802–2807 (1967).
Takahara, S., Yamamuro, O. & Suga, H. Heat capacities and glass transitions of 1-propanol and 3-methyl pentane: new evidence for the entropy theory. J. Non-Cryst. Solids 171, 259–270 (1994).
Takahara, S., Yamamuro, O. & Matsuo, T. Calorimetric study of 3-bromopentane—correlation between structural relaxation time and configurational entropy. J. Phys. Chem. 99, 9580–9592 (1995).
Angell, C. A. Liquid landscapes. Nature 393, 521–522 (1998).
Starr, F. W. et al. Thermodynamic and structural aspects of the potential energy surface of simulated water. Phys. Rev. E. (in the press); also preprint arXiv:cond-mat/0007487 on http://xxx.lanl.gov/ (2000).
Phillips, W. A., Buchenau, U., Nücker, N., Dianou, A. J. & Petry, W. Dynamics of glassy and liquid selenium. Phys. Rev. Lett. 63, 2381–2384 (1989).
Wischnewski, A., Buchenau, U., Dianoux, A. J., Kamitakahara, W. A. & Zarestky, J. L. Neutron scattering analysis of low-frequency modes in silica. Phil. Mag. B 77, 579–589 (1998).
Kob, W., Sciortino, F. & Tartaglia, P. Aging as dynamics in configuration space. Europhys. Lett. 49, 590–596 (2000).
Acknowledgements
We thank S. Sastry, R. Speedy and F. Sciortino for comments and criticisms. This work was supported by the NSF, Division of Materials Research, Solid State Chemistry program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez, LM., Angell, C. A thermodynamic connection to the fragility of glass-forming liquids. Nature 410, 663–667 (2001). https://doi.org/10.1038/35070517
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35070517
This article is cited by
-
Electromagnetic levitation containerless processing of metallic materials in microgravity: rapid solidification
npj Microgravity (2023)
-
Developing novel amorphous alloys from the perspectives of entropy and shear bands
Science China Materials (2023)
-
Structural Relaxation and Thermodynamics of Viscous Aqueous Systems: A Simplified Reappraisal
Journal of Solution Chemistry (2023)
-
Compositional dependence of the fragility in metallic glass forming liquids
Nature Communications (2022)
-
Disentangling structural and kinetic components of the α-relaxation in supercooled metallic liquids
Communications Physics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.