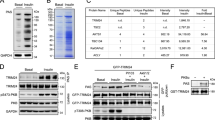
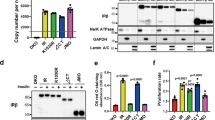
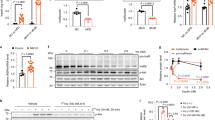
Abstract
One of the critical responses to insulin treatment is the stimulation of protein synthesis through induced phosphorylation of the eIF-4E-binding protein 1 (4E-BP1), and the subsequent release of the translation initiation factor, eIF-4E. Here we report that ATM, the protein product of the ATM gene that is mutated in the disease ataxia telangiectasia, phosphorylates 4E-BP1 at Ser 111 in vitro and that insulin treatment induces phosphorylation of 4E-BP1 at Ser 111 in vivo in an ATM-dependent manner. In addition, insulin treatment of cells enhances the specific kinase activity of ATM. Cells lacking ATM kinase activity exhibit a significant decrease in the insulin-induced dissociation of 4E-BP1 from eIF-4E. These results suggest an unexpected role for ATM in an insulin-signalling pathway that controls translation initiation. Through this mechanism, a lack of ATM activity probably contributes to some of the metabolic abnormalities, such as poor growth and insulin resistance, reported in ataxia telangiectasia cells and patients with ataxia telangiectasia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Canman, C. E. & Lim, D. S. The role of ATM in DNA damage responses and cancer. Oncogene 17, 3301–3308 (1998).
Lavin, M. F. & Shiloh, Y. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15, 177–202 (1997).
Elson, A. et al. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl Acad. Sci. USA 93, 13084 –13089 (1996).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 ( 1996).
Bar, R. S. et al. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N. Engl. J. Med. 298 , 1164–1171 (1978).
Schalch, D. S., McFarlin, D. E. & Barlow, M. H. An unusual form of diabetes mellitus in ataxia telangiectasia. N. Engl. J. Med. 282, 1396–1402 (1970).
Blevins, L. S. Jr & Gebhart, S. S. Insulin-resistant diabetes mellitus in a black woman with ataxia-telangiectasia . South. Med. J. 89, 619– 621 (1996).
Morgan, S. E. & Kastan, M. B. p53 and ATM: cell cycle, cell death, and cancer. Adv. Cancer Res. 71, 1–25 (1997).
Kastan, M. B. et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71, 587–597 (1992).
Xu, Y. & Baltimore, D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 10, 2401–2410 (1996).
Shiloh, Y., Tabor, E. & Becker, Y. Abnormal response of ataxia-telangiectasia cells to agents that break the deoxyribose moiety of DNA via a targeted free radical mechanism. Carcinogenesis 4, 1317– 1322 (1983).
Elmore, E. & Swift, M. Growth of cultured cells from patients with ataxia-telangiectasia. J. Cell Physiol. 89, 429–431 (1976).
Thomas, G. & Hall, M. N. TOR signalling and control of cell growth. Curr. Opin. Cell Biol. 9, 782–787 (1997).
Brown, E. J. & Schreiber, S. L. A signaling pathway to translational control. Cell 86, 517– 520 (1996).
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749 –1753 (1995).
Canman, C. E. et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677– 1679 (1998).
Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage . Science 281, 1674–1677 (1998).
Cortez, D., Wang, Y., Qin, J. & Elledge, S. J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286, 1162– 1166 (1999).
Zhou, B. B. et al. Caffeine abolishes the mammalian G2/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275, 10342–10348 (2000).
Lim, D. S. et al. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway . Nature 404, 613–617 (2000).
Sarkaria, J. N. et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 58, 4375–4382 (1998).
Chan, D. W. et al. Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase . J. Biol. Chem. 275, 7803– 7810 (2000).
Gingras, A. C. et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism . Genes Dev. 13, 1422–1437 (1999).
Yang, D., Brunn, G. J. & Lawrence, J. C. Jr Mutational analysis of sites in the translational regulator, PHAS-I, that are selectively phosphorylated by mTOR . FEBS Lett. 453, 387–390 (1999).
Burnett, P. E., Barrow, R. K., Cohen, N. A., Snyder, S. H. & Sabatini, D. M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl Acad. Sci. USA 95, 1432–1437 (1998).
Hara, K. et al. Regulation of eIF-4E BP1 phosphorylation by mTOR. J. Biol. Chem. 272, 26457–26463 (1997).
Brunn, G. J. et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 (1997).
Lin, T. A. et al. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266, 653–656 (1994).
Pause, A. et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature 371, 762–767 (1994).
Sonenberg, N. & Gingras, A. C. The mRNA 5' cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol. 10, 268–275 ( 1998).
Lawrence, J. C. Jr & Abraham, R. T. PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem. Sci. 22, 345–349 (1997).
Proud, C. G. & Denton, R. M. Molecular mechanisms for the control of translation by insulin. Biochem. J. 328, 329–341 (1997).
Kim, S. T., Lim, D. S., Canman, C. E. & Kastan, M. B. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 274, 37538 –37543 (1999).
Heesom, K. J., Avison, M. B., Diggle, T. A. & Denton, R. M. Insulin-stimulated kinase from rat fat cells that phosphorylates initiation factor 4E-binding protein 1 on the rapamycin-insensitive site (serine-111) . Biochem. J. 336, 39–48 (1998).
Brown, E. J. et al. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377, 441– 446 (1995).
Diggle, T. A. et al. Both rapamycin-sensitive and -insensitive pathways are involved in the phosphorylation of the initiation factor-4E-binding protein (4E-BP1) in response to insulin in rat epididymal fat-cells. Biochem. J. 316, 447–453 ( 1996).
Beretta, L., Gingras, A. C., Svitkin, Y. V., Hall, M. N. & Sonenberg, N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation . EMBO J. 15, 658–664 (1996).
Mothe-Satney, I., Yang, D., Fadden, P., Haystead, T. A. & Lawrence, J.C. Jr Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol. Cell. Biol. 20, 3558–3567 (2000).
Fadden, P., Haystead, T. A. & Lawrence, J. C. Jr Identification of phosphorylation sites in the translational regulator, PHAS-I, that are controlled by insulin and rapamycin in rat adipocytes. J. Biol. Chem. 272 , 10240–10247 (1997).
Fadden, P., Haystead, T. A. & Lawrence, J. C. Jr Phosphorylation of the translational regulator, PHAS-I, by protein kinase CK2. FEBS Lett. 435, 105–109 (1998).
Westphal, C. H. et al. Genetic interactions between atm and p53 influence cellular proliferation and irradiation-induced cell cycle checkpoints. Cancer Res. 57, 1664–1667 (1997).
Brown, K. D. et al. The ataxia-telangiectasia gene product, a constitutively expressed nuclear protein that is not up-regulated following genome damage . Proc. Natl Acad. Sci. USA 94, 1840– 1845 (1997).
Lakin, N. D. et al. Analysis of the ATM protein in wild-type and ataxia telangiectasia cells. Oncogene 13, 2707– 2716 (1996).
Lim, D. S. et al. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc. Natl Acad. Sci. USA 95, 10146– 10151 (1998).
Watters, D. et al. Localization of a portion of extranuclear ATM to peroxisomes . J. Biol. Chem. 274, 34277– 34282 (1999).
Barlow, C. et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc. Natl Acad. Sci. USA 97 , 871–876 (2000).
Barlow, C. et al. ATM deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 125, 4007–4017 (1998).
Oka, A. & Takashima, S. Expression of the ataxia-telangiectasia gene (ATM) product in human cerebellar neurons during development. Neurosci. Lett. 252, 195–198 (1998).
Tuhackova, Z., Sovova, V., Sloncova, E. & Proud, C. G. Rapamycin-resistant phosphorylation of the initiation factor-4E-binding protein (4E-BP1) in v-SRC-transformed hamster fibroblasts. Int. J. Cancer 81, 963–969 ( 1999).
Poulin, F., Gingras, A. C., Olsen, H., Chevalier, S. & Sonenberg, N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 273, 14002–14007 (1998).
Acknowledgements
We thank T. M. Gilmer and Y. Shiloh for monoclonal antibodies to ATM; D. Sabatini for 4E-BP1 and eIF-4E cDNA reagents; P. Leder for AT-null murine fibroblasts; A. Friedman for Mouse 3T3 L1 cells. We also thank P. Houghton and J. C. Jones for helpful comments on the manuscript, and C. Canman, D-S. Lim, S-T. Kim, D. Woods and A. Justman for advice and technical assistance. This work was supported by grants from the NIH. M.B.K. is also supported by the American Lebanese Syrian Associated Charities (ALSAC) of the St. Jude Children's Research Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, DQ., Kastan, M. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol 2, 893–898 (2000). https://doi.org/10.1038/35046542
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35046542
This article is cited by
-
DNA damage alters EGFR signaling and reprograms cellular response via Mre-11
Scientific Reports (2022)
-
Deletion of translin (Tsn) induces robust adiposity and hepatic steatosis without impairing glucose tolerance
International Journal of Obesity (2020)
-
Functional interplay between the oxidative stress response and DNA damage checkpoint signaling for genome maintenance in aerobic organisms
Journal of Microbiology (2020)
-
Regulation of DNA damage-induced ATM activation by histone modifications
Genome Instability & Disease (2020)
-
ATM, DNA-PKcs and ATR: shaping development through the regulation of the DNA damage responses
Genome Instability & Disease (2020)