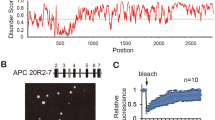
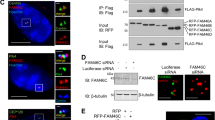
Abstract
The adenomatous polpyposis coli (APC) protein is mutated in most colorectal tumours1. Nearly all APC mutations are truncations, and many of these terminate in the mutation cluster region located halfway through the protein2,3,4. In cancer cells expressing mutant APC, β-catenin is stabilized5,6 and translocates into the nucleus to act as a transcriptional co-activator of T-cell factor7. During normal development, APC also promotes the destabilization of β-catenin and Drosophila Armadillo8,9,10,11. It does so by binding to the Axin complex which earmarks β-catenin/Armadillo for degradation by the proteasome pathway12. APC has a regulatory role in this process13,14, which is poorly understood. Here we show that APC contains highly conserved nuclear export signals 3′ adjacent to the mutation cluster region that enable it to exit from the nucleus. This ability is lost in APC mutant cancer cells, and we provide evidence that β-catenin accumulates in the nucleus as a result. Thus, the ability of APC to exit from the nucleus appears to be critical for its tumour suppressor function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Nagase, H. & Nakamura, Y. Mutations of the APC (Adenomatous polyposis coli) gene. Hum. mutat. 2, 425 –434 (1993).
Miyaki, M. et al. Characteristics of somatic mutation of the Adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 54, 3011–3020 (1994).
Lamlum, H. et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's ‘two-hit’ hypothesis. Nature Med. 5, 1071–1075 (1999).
Munemitsu, S., Albert, I., Souza, B., Rubinfeld, B. & Polakis, P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl Acad. Sci. USA 92, 3046– 3050 (1995).
Morin, P. J. et al. Activation of β-catenin-TCF signaling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science 275, 1784–1787 (1997).
Ahmed, Y., Hayashi, S., Levine, A. & Wieschaus, E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171– 1182 (1998).
Yu, X., Waltzer, L. & Bienz, M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nature Cell Biol. 3, 144–151 (1999).
McCartney, B. M. et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 146, 1303–1318 (1999).
Farr, G. H. et al. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J. Cell. Biol. 148, 691–702 (2000).
Peifer, M. & Polakis, P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287, 1606–1609 (2000).
Behrens, J. et al. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596– 599 (1998).
Hart, M. J., de los Santos, R., Albert, I. N., Rubinfeld, B. & Polakis, P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr. Biol. 8, 573– 581 (1998).
Neufeld, K. L. & White, R. L. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc. Natl Acad. Sci. USA 94, 3034–3039 (1997).
Fagotto, F., Gluck, U. & Gumbiner, B. M. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 8, 181–190 (1998).
Yokoya, F., Imamoto, N., Tachibana, T. & Yoneda, Y. β-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol. Biol. Cell 10, 1119– 1131 (1999).
Fukuda, M. et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308 –311 (1997).
Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051–1060 (1997).
Kudo, N. et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540–547 (1998).
Su, L. K., Vogelstein, B. & Kinzler, K. W. Association of the APC tumor suppressor protein with catenins. Science 262, 1734– 1737 (1993).
Rubinfeld, B., Albert, I., Porfiri, E., Munemitsu, S. & Polakis, P. Loss of β-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res. 57, 4624– 4630 (1997).
Smits, R. et al. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 13, 1309–1321 (1999).
Shih, I. M., Yu, J., He, T. C., Vogelstein, B. & Kinzler, K. W. The β-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 60, 1671–1676 ( 2000).
Lal, G. & Gallinger, S. Familial adenomatous polyposis. Sem. Surg. Onco. 18, 314– 323 (2000).
Rowan, A. J. et al. APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc. Natl Acad. Sci. USA 97, 3352– 3357 (2000).
Näthke, I. S., Adams, C. L., Polakis, P., Sellin, J. H. & Nelson, W. J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134, 165– 179 (1996).
Hamada, F. et al. Identification and characterization of E-APC, a novel Drosophila homologue of the tumour suppressor APC. Genes Cells 4, 465–474 ( 1999).
Bienz, M. APC: the plot thickens. Curr. Opin. Genet. Dev. 9, 595–603 (1999).
Lantz, V. A., Clemens, S. E. & Miller, K. G. The actin cytoskeleton is required for maintenance of posterior pole plasm components in the Drosophila embryo. Mech. Dev. 85, 111–122 ( 1999).
Acknowledgements
We thank M. Yoshida for LMB; I. Näthke for APC antibody; T. Brabletz and J. Behrens for colon cancer cells; H. Clevers for plasmids; M. West for help with the luciferase assays; C. Garvey for help with the Western blots; A. Cliffe for NES searches; and R. Arkowitz, T. Brabletz, F. Fagotto and W. Bodmer for discussion. R.R.-A. is supported by a Wellcome travelling fellowship.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Rosin-Arbesfeld, R., Townsley, F. & Bienz, M. The APC tumour suppressor has a nuclear export function. Nature 406, 1009–1012 (2000). https://doi.org/10.1038/35023016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35023016
This article is cited by
-
Palmitoyltransferase DHHC9 and acyl protein thioesterase APT1 modulate renal fibrosis through regulating β-catenin palmitoylation
Nature Communications (2023)
-
Identifying novel interactions of the colon-cancer related APC protein with Wnt-pathway nuclear transcription factors
Cancer Cell International (2022)
-
Wnt/β-catenin signaling in cancers and targeted therapies
Signal Transduction and Targeted Therapy (2021)
-
Multivalent tumor suppressor adenomatous polyposis coli promotes Axin biomolecular condensate formation and efficient β-catenin degradation
Scientific Reports (2020)
-
APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.