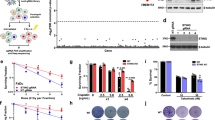
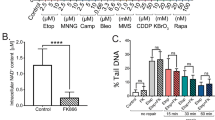
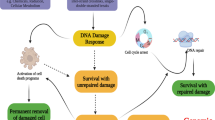
Abstract
Nitric-oxide synthase (NOS) activity has been detected in many human tumours, although its function is unclear. Here we show that exposure of cells to nitric oxide (NO) results in a 4–5-fold increase in expression of the DNA-dependent protein-kinase catalytic subunit (DNA-PKcs), one of the key enzymes involved in repairing double-stranded DNA breaks. This NO-mediated increase in enzymatically active DNA-PK not only protects cells from the toxic effects of NO, but also provides crossprotection against clinically important DNA-damaging agents, such as X-ray radiation, adriamycin, bleomycin and cisplatin. The NO-mediated increase in DNA-PKcs described here demonstrates the presence of a new and highly effective NO-mediated mechanism for DNA repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Moncada, S., Palmer, R. M. & Higgs, E. A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 43, 109–142 (1991).
Knowles, R. G. & Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 298, 249–258 (1994).
Jenkins, D. C. et al. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Brit. J. Cancer 70, 847–849 (1994).
Asano, K. et al. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl Acad. Sci. USA 91, 10089–10093 (1994).
Ambs, S. et al. Frequent nitric oxide synthase-2 expression in human adenomas: implication for tumour angiogenesis and colon cancer progression. Cancer Res. 58, 334–341 (1998).
Thomsen, L. L., Sargent, J. M., Williamson, C. J. & Elgie, A. W. Nitric oxide synthase activity in fresh cells from ovarian tumour tissue: relationship of enzyme activity with clinical parameters of patients with ovarian cancer. Biochem. Pharmacol. 56, 1365–1370 (1998).
Zhao, H. et al. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity. Blood 92, 1031–1043 (1998).
Jenkins, D. C. et al. Roles of nitric oxide in tumour growth. Proc. Natl Acad. Sci. USA 92, 4392–4396 (1995).
Forrester, K. et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc. Natl Acad. Sci. USA 93, 2442–2447 (1996).
Ambs, S. et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nature Med. 4, 1371–1376 (1998).
Burney, S., Caulfield, J. L., Niles, J. C., Wishnok, J. S., & Tannenbaum, S. R. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 424, 37–49 (1999).
Smith, G. C. M. & Jackson, S. P. The DNA-dependent protein kinase. Genes Dev. 13, 916–934 (1999).
No, D., Yao, T. P. & Evans, R. M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl Acad. Sci. USA 93, 3346–3351 (1996).
Charles, I. G. et al. Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc. Natl Acad. Sci. USA 90, 11419–11423 (1993).
Salter, M., Knowles, R. G. & Moncada, S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 291, 145–149 (1991).
Hartley, K. O. et al. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell 82, 849–856 (1995).
Song, Q. et al. DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 15, 3238–3246 (1996).
Finnie, N. J., Gottlieb, T. M., Blunt, T., Jeggo, P. A. & Jackson, S. P. DNA-dependent protein kinase activity is absent in xrs-6 cells: implications for site specific recombination and DNA double strand break repair. Proc. Natl Acad. Sci. USA 92, 320–324 (1995).
Connelly, M. A., Zhang, H., Kieleczawa, J. & Anderson, C. W. The promoters for human DNA-PKcs (PRKDC) and MCM 4: divergently transcribed genes located at chromosome 8 band q 11. Genomics 47, 71–83 (1998).
Darius, H. et al. The effects of molsidomine and its metabolite SIN-1 on coronary vessel tone, platelet aggregation, and eicosanoid formation in vitro — inhibition of 12-HPETE biosynthesis. J. Cardiovasc. Pharmacol. 6, 115–121 (1984).
Courey, A. J. & Tjian, R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55, 887–898 (1988).
Lees-Miller, S. P. et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science 267, 1183–1185 (1995).
Izzard, R. A., Jackson, S. P. & Smith, G. C. Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res. 59, 2581–2586 (1999).
Woudstra, E. C., Driessen, C., Konings, A. W. & Kampinga, H. H. DNA damage induction and tumour cell radiosensitivity: PFGE and halo measurements. Int. J. Radiat. Biol. 73, 495–502 (1998).
Allalunis-Turner, M. J. et al. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat. Res. 134, 349–354 (1993).
Muller, C. et al. UV sensitivity and impaired nucleotide excision repair in DNA-dependent protein kinase mutant cells. Nucleic Acids Res. 26, 1382–1389 (1998).
Shen, H., Schultz, M., Kruh, G. D. & Tew, K. D. Increased expression of DNA-dependent protein kinase confers resistance to adriamycin. Biochimica et Biophysica Acta 1381, 131–138 (1998).
Muller, C., Christodoulopoulos, G., Salles, B. & Panasci, L. DNA-dependent protein kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood 92, 2213–2219 (1998).
Suschek, C. V. et al. Nitric oxide fully protects against UVA-induced apoptosis in tight correlation with Bcl-2 upregulation. J. Biol. Chem. 274, 6130–6137 (1999).
Dignam, J. D., Lebovitz, R. M. & Roeder, R. G. Accurate transcription initiation by RNA polymerase-II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983).
Yagle, M. K., Parruti, G., Xu, W., Ponder, B. & Solomon, E. Genetic and physical map of the von Recklinghausen neurofibromatosis (NF1) region on chromosome 17. Proc. Natl Acad. Sci. USA 87, 7255–7259 (1990).
Acknowledgements
We thank C. Jenkins and S. Jackson for discussions, C. W. Anderson (Brookhaven National Laboratory, New York) for DNA-PKcs promoter–reporter plasmids, R. Tjian (Howard Hughes Medical Institute, Berkeley, Illinois) for pPacSp1 and pPacO plasmids and M. J. Allalunis-Turner for cell lines M059J and M059K. We also thank D. Sheer for help in the use of the X-ray facility at the ICRF, London. This work was supported by a grant from the Medical Research Council.
Correspondence and requests for materials should be addressed to I.G.C.
Supplementary information is available on Nature Cell Biology’s website (http://www.nature.com/ncb) or as paper copy from the London editorial office of Nature Cell Biology.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information
Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. (PDF 34 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Liu, L., Smith, G. et al. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat Cell Biol 2, 339–345 (2000). https://doi.org/10.1038/35014028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35014028
This article is cited by
-
Development and evaluation of an expedited system for creation of single walled carbon nanotube platforms
Carbon Letters (2024)
-
Significance of twist and iNOS expression in human breast carcinoma
Molecular and Cellular Biochemistry (2016)
-
Inhibition of DNA-dependent protein kinase catalytic subunit by small molecule inhibitor NU7026 sensitizes human leukemic K562 cells to benzene metabolite-induced apoptosis
Journal of Huazhong University of Science and Technology [Medical Sciences] (2013)
-
Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies
Cancer Chemotherapy and Pharmacology (2011)
-
DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals
Oncogene (2008)