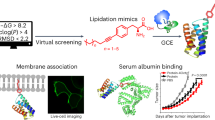
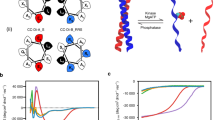
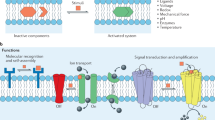
Abstract
Biological membranes define the boundaries of the cellular compartments in higher eukaryotes and are active in many processes such as signal transduction and vesicular transport. Although post-translational lipid modification of numerous proteins in signal transduction is crucial for biological function1, analysis of protein–protein interactions has mainly focused on recombinant proteins in solution under defined in vitro conditions. Here we present a new strategy for the synthesis of such lipid-modified proteins. It involves the bacterial expression of a carboxy-terminally truncated non-lipidated protein, the chemical synthesis of differently lipidated peptides representing the C terminus of the proteins, and their covalent coupling. Our technique is demonstrated using Ras constructs, which exhibit properties very similar to fully processed Ras, but can be produced in high yields and are open for selective modifications. These constructs are operative in biophysical and cellular assay systems, showing specific recognition of effectors by Ras lipoproteins inserted into the membrane surface of biosensors and transforming activity of oncogenic variants after microinjection into cultured cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Resh, M. D. Regulation of cellular signalling by fatty acid acylation and prenylation of signal transduction proteins. Cell. Signal. 8, 403–412 (1996).
Wittinghofer, A. Signal transduction via Ras. Biol. Chem. 379, 933–973 (1998).
Gelb, M. H. Protein prenylation, et cetera: signal transduction in two dimensions. Science 275, 1750–1751 (1997).
Waddick, K. G. & Uckun, F. M. Innovative treatment programs against cancer. I. Ras oncoprotein as a molecular target. Biochem. Pharmacol. 56, 1411–1426 (1998).
Page, M. J. et al. Expression and characterization of the Ha-ras p21 protein produced at high levels in the insect/baculovirus system. J. Biol. Chem. 264, 19147–19154 (1989).
Schmittberger, T., Cotté, A. & Waldmann, H. Synthesis of characteristic lipopeptides of lipid modified proteins employing the allylester as protecting group. Chem. Comm., 937–938 (1998).
Nagele, E., Schelhaas, M., Kuder, N. & Waldmann, H. Chemoenzymatic synthesis of N-Ras lipopeptides. J. Am. Chem. Soc. 120, 6889–6902 (1998).
Tucker, J. et al. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 5, 1351–1358 (1986).
Bordier, C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256, 1604–1607 (1981).
Kalb, E., Frey, S. & Tamm, L. K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta 1103, 307–316 (1992).
Schelhaas, M. et al. Chemoenzymatic synthesis of biotinylated ras peptides and their use in membrane binding studies of lipidated model proteins by surface plasmon resonance. Chem. Eur. J. 5, 1239–1252 (1999).
Herrmann, C., Martin, G. A. & Wittinghofer, A. Quantitative analysis of the complex between p21ras and the Ras-binding domain of the human Raf-1 protein kianse. J. Biol. Chem. 270, 2901–2905 (1995).
Lenzen, C., Cool, R. H., Prinz, H., Kuhlmann, J. & Wittinghofer, A. Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry 37, 7420–7930 (1998).
Bar-Sagi, D. & Feramisco, J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42, 841–848 (1985).
Schmidt, G. et al. Biochemical and biological consequences of changing the specificity of p21ras from guanosine to xanthosine nucleotides. Oncogene 12, 87–96 (1996).
Willumsen, B. M., Cox, A. D., Solski, P. A., Der, C. J. & Buss, J. E. Novel determinants of H-Ras plasma membrane localization and transformation. Oncogene 13, 1901–1909 (1996).
Siddiqui, A. A., Garland, J. R., Dalton, M. B. & Sinensky, M. Evidence for a high affinity, saturable, prenylation-dependent p21Ha-ras binding site in plasma membranes. J. Biol. Chem. 273, 3712–3717 (1998).
Shahinian, S. & Silvius, J. R. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry 34, 3813–3822 (1995).
MacDonald, R. C. et al. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1061, 297–303 (1991).
Mayer, L. D., Hope, M. J. & Cullis, P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 858, 161–168 (1986).
Schelhaas, M., Glomsda, S., Hänsler, M., Jakubke, H. D. & Waldmann, H. Enzymatic synthesis of peptides and Ras lipopeptides employing choline ester as a solubilizing, protecting, and activating group. Angew. Chem. Int. Edn Engl. 35, 106–109 (1996).
Acknowledgements
We thank C. Nowak for technical support, D. Vogt for cloning C-terminally truncated H-Ras and H. Prinz for mass spectroscopical analysis. This research was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bader, B., Kuhn, K., Owen, D. et al. Bioorganic synthesis of lipid-modified proteins for the study of signal transduction. Nature 403, 223–226 (2000). https://doi.org/10.1038/35003249
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35003249
This article is cited by
-
Myr-Arf1 conformational flexibility at the membrane surface sheds light on the interactions with ArfGAP ASAP1
Nature Communications (2023)
-
Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials
Nature Chemistry (2018)
-
Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing
BMC Genomics (2014)
-
Lipidated peptides via post-synthetic thioalkylation promoted by molecular sieves
Amino Acids (2014)
-
Small-molecule inhibition of APT1 affects Ras localization and signaling
Nature Chemical Biology (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.