Abstract
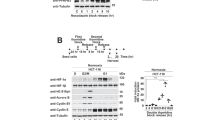
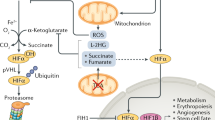
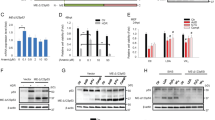
Although hypoxia (lack of oxygen in body tissues) is perhaps the most physiological inducer of the wild-type p53 gene1, the mechanism of this induction is unknown. Cells may detect low oxygen levels through a haem-containing sensor protein2. The hypoxic state can be mimicked by using cobalt chloride and the iron chelator desferrioxamine2,3,4,5: like hypoxia, cobalt chloride and desferrioxamine activate hypoxia-inducible factor 1α (HIF-1α) (ref. 6), which stimulates the transcription of several genes that are associated with hypoxia6,7,8,9. Here we show that these treatments induce accumulation of wild-type p53 through HIF-1α-dependent stabilization of p53 protein. Induction of p53 does not occur in either a mutant hepatoma cell line that is unable to induce HIF-1α (ref. 10) or embryonic stem cells derived from mice lacking HIF-1β (ref. 11). HIF-1α is found in p53 immunoprecipitates from MCF7 cells that express wild-type p53 and are either hypoxic or have been exposed to desferrioxamine. Similarly, anti-haemagglutinin immunoprecipitates from lysates of normoxic PC3M cells that had been co-transfected with haemagglutinin-tagged HIF-1α and wild-type p53 also contain p53. Transfection of normoxic MCF7 cells with HIF-1α stimulates a co-transfected p53-dependent reporter plasmid and increases the amount of endogenous p53. Our results suggest that hypoxic induction of transcriptionally active wild-type p53 is achieved as a result of the stabilization of p53 by its association with HIF-1α.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Graeber, T. G. et al. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14, 6264–6277 (1994).
Goldberg, M. A., Dunning, S. P. & Bunn, H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242, 1412–1415 (1988).
Salceda, S., Beck, I. & Caro, J. Absolute requirement of aryl hydrocarbon receptor nuclear translocator protein for gene activaiton by hypoxia. Arch. Biochem. Biophys. 384, 389–394 (1996).
Gradin, K. et al. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol. Cell Biol. 16, 5221–5231 (1996).
Guillemin, K. & Krasnow, M. A. The hypoxic response: huffing and HIFing. Cell 89, 9–12 (1997).
Wang, G. L. & Semenza, G. L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implicaitons for model of hypoxia signal transduction. Blood 82, 3610–3615 (1993).
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Minchenko, A., Salceda, S., Bauer, T. & Caro, J. Hypoxia regulatory elements of the human vascular endothelial growth factor gene. Cell Mol. Biol. Res. 40, 35–39 (1994).
Levy, A. P., Levy, N. S., Wegner, S. & Goldberg, M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem. 270, 13333–13340 (1995).
Hoffman, E. C. et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 (1991).
Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A. & Simon, M. C. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 (1997).
Wang, G. L. & Semenza, G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl Acad. Sci. USA 90, 4304–4308 (1993).
Wang, G. L., Jiang, B.-H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Wang, G. L. & Semenza, G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995).
Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. J. Biol. Chem. 271, 15117–15123 (1996).
Kallio, P. J., Pongratz, I., Gradin, K., McGuire, J. & Poellinger, L. Activaiton of hypoxia-inducible factor 1α: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl Acad. Sci. USA 94, 5667–5672 (1997).
Maki, C. G., Huibregtse, J. & Howley, P. M. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 56, 2649–2654 (1996).
Whitesell, L. et al. Geldanamycin-stimulated destabilization of mutated p53 is mediated by the proteasome in vivo. Oncogene 14, 2809–2816 (1997).
Andrews, N. & Faller, D. Arapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids. Res. 19, 2499 ((1991)).
Blagosklonny, M., Toretsky, J. & Neckers, L. Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 11, 933–939 (1995).
Arany, Z. et al. An essential role for p300/CPB in the cellular response to hypoxia. Proc. Natl Acad. Sci. USA 93, 12969–12973 (1996).
Huang, L. E., Arany, Z., Livingston, D. M. & Bunn, H. F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. J. Biol. Chem. 271, 32253–32259 (1996).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Acknowledgements
We thank J. Trepel and O. Hankinson for cell lines, and B. Vogelstein and D.Livingston for plasmids and antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, W., Kanekal, M., Simon, M. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392, 405–408 (1998). https://doi.org/10.1038/32925
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/32925
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.