Abstract
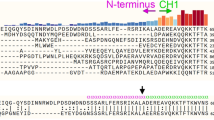
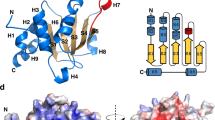
THE ‘pleckstrin homology’ or PH domain is a 100-residue protein module. It is present in many kinases, different isoforms of phospholipase C, GTPase-activating proteins and nucleotide-exchange factors1–4. Its function is not known, but many proteins that contain a PH domain interact with GTP-binding proteins5. The PH domain in β-adrenergic receptor kinase may be involved in binding to the βγ subunits of a trimeric G-protein3, 4, 6, 7. We report here the three-dimensional structure of the PH domain of the cytoskeletal protein spectrin using homonuclear nuclear magnetic resonance. The core of the molecule is an antiparallel β-sheet consisting of seven strands. The C terminus is folded into a long α-helix, and another helix is present in one of the surface loops. The molecule is electrostatically polarized and contains a pocket which may be involved in the binding of a ligand. There is a distant relationship to the peptidyl-prolyl-cis-trans-isomerase FKBP in which this pocket is involved in the binding of the macrocyclic compound FK506(refs 8–11).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Haslam, R. L., Kolde, H. B. & Hemmings, B. A. Nature 363, 309–310 (1993).
Meyer, B. J., Ren, R., Clark, K. L. & Baltimore, D. Cell 73, 629–630 (1993).
Musacchio, A., Gibson, T., Rice, P., Thompson, J. & Saraste, M. Trends biochem. Sci. 18, 343–348 (1993).
Shaw, G. Biochem. biophys. Res. Commun. 195, 1145–1151 (1993).
Boguski, M. S. & McCormick, F. Nature 366, 643–654 (1993).
Inglese, J., Kech, W. J., Caron, M. G. & Lefkowitz, R. J. Nature 359, 147–150 (1992).
Koch, W. J., Inglese, J., Stone, W. C. & Lefkowitz, R. J. J. biol. Chem. 268, 8256–8260 (1993).
Standaert, R. F., Galat, A., Verdine, G. L. & Schreiber, S. L. Nature 346, 671–674 (1990).
Topschug, M., Wachter, E., Mayer, S., Schönbrunner, E. R. & Schmid, F. X. Nature 346, 674–677 (1990).
Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. Science 252, 839–842 (1991).
Meadows, R. P. et al. Biochemistry 32, 754–765 (1993).
Aue, W. P., Bartholdi, E. & Ernst, R. R. J. chem. Phys. 64, 2229–2246 (1976).
Giesinger, C., Otting, G., Wütrich, K. & Ernst, R. R. J. Am. chem. Soc. 110, 7870–7871 (1988).
Jeener, J., Meier, B. H., Bachmann, P. & Ernst, R. R. J. chem. Phys. 71, 4546–4553 (1979).
Oschkinat, H. et al. Nature 332, 374–376 (1988).
Wu, D., Katz, A. & Simon, M. I. Proc. natn. Acad. Sci. U.S.A. 90, 5297–5301 (1993).
Jiang, H., Wu, D. & Simon, M. I. J. biol. Chem. 269, 7593–7596 (1994).
Parker, P. J., Hemmings, B. A. & Gierschik, P. Trends biochem. Sci. 19, 54–55 (1994).
Davis, L. H. & Bennett, V. J. biol. Chem. 269, 4409–4416 (1994).
Thomas, J. D. et al. Science 261, 355–358 (1993).
Rawlings, D. J. et al. Science 261, 358–361 (1993).
Holm, L. & Sander, C. J. molec. Biol. 233, 123–138 (1993).
Musacchio, A., Noble, M., Pauptit, R., Wierenga, R. & Saraste, M. Nature 359, 851–855 (1992).
Kraulis, P. J. appl. Crystallogr. 24, 946–950 (1991).
Brünger, A. T. X-PLOR, Version 3.1, A System for X-Ray Crystallography and NMRR (Yale Univ. Press, New Haven, London, 1992).
Nilges, M., Kuszewski, J. & Brünger, A. T. in Computational Aspects of the Study of Biological Macromolecules by Nuclear Magnetic Resonance Spectroscopy (eds Hoch, J. C., Poulsen, F. M. & Redfield, C.) 451–455 (Plenum, New York, 1991).
Nilges, M. Proteins 17, 297–309 (1993).
Gilson, M. K., Sharp, K. A. & Honig, B. J. Comput. Chem. 9, 327–335 (1988).
Nicholls, A., Sharp, K. A. & Honig, B. Proteins 11, 281–296 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Macias, M., Musacchio, A., Ponstingl, H. et al. Structure of the pleckstrin homology domain from β-spectrin. Nature 369, 675–677 (1994). https://doi.org/10.1038/369675a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/369675a0
This article is cited by
-
Human erythrocytes: cytoskeleton and its origin
Cellular and Molecular Life Sciences (2020)
-
Computational studies of the binding profile of phosphoinositide PtdIns (3,4,5) P3 with the pleckstrin homology domain of an oomycete cellulose synthase
Scientific Reports (2016)
-
Syntrophin proteins as Santa Claus: role(s) in cell signal transduction
Cellular and Molecular Life Sciences (2013)
-
The spectrin–ankyrin–4.1–adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life
Protoplasma (2010)
-
Structure of the split PH domain and distinct lipid-binding properties of the PH-PDZ supramodule of α-syntrophin
The EMBO Journal (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.