Abstract
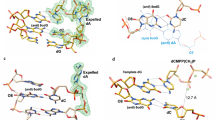
The cutting rates of bovine pancreatic deoxyribonuclease I (DNase I) vary along a given DNA sequence, indicating that the enzyme recognizes sequence-dependent structural variations of the DNA double-helix1,2. In an attempt to define the helical parameters determining this sequence-dependence, we have co-crystallized a complex of DNase I with a self-complementary octanucleotide and refined the crystal structure at 2 Å resolution. This structure confirms the basic features of an early model3,4, namely that an exposed loop of DNase I binds in the minor groove of B-type DNA and that interactions do occur with the backbone of both strands. Nicked octamer duplexes that have lost a dinucleotide from the 3′-end of one strand are hydrogen-bonded across a two-fold axis in the crystal to form a quasi-continuous double helix of 14 base pairs. The DNA 14-mer has a B-type conformation and shows substantial distortion of both local and overall helix parameters, induced mainly by the tight interaction of Y73 and R38 in the unusually wide minor groove. Directly coupled to the widening of the groove by ∼3Å is a 21.5° bend of the DNA away from the bound enzyme towards the major groove, suggesting that both DNA stiffness and groove width are important in determining the sequence-dependence of the enzyme cutting rate. A second cut of the DNA which is induced by diffusion of Mn2+ into the co-crystals suggests that there are two active sites in DNase I separated by more than 15Å.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lomonossoff, G. P., Butler, P. J. G. & Klug, A. J. molec. Biol. 149, 745–760 (1981).
Drew, H. R. & Travers, A. A. Cell 37, 491–502 (1984).
Oefner, C. & Suck, D. J. molec. Biol. 192, 605–632 (1986).
Suck, D. & Oefner, C. Nature 312, 620–625 (1986).
Rossmann, M. G. (ed.) The Molecular Replacement Method (Gordon & Breach, New York, 1972).
Hendrickson, W. A. & Konnert, J. H. in Computing in Crystallography (eds Diamond, R. Ramaseshan, S. & Ventkatesan, K.) 13.01–13.23 (Indian Academy of Science, Bangalore, 1980).
Price, P. A., Stein, W. H. & Moore, S. J. biol. Chem. 244, 929–932 (1969).
Dickerson, R. E. Scient. Am. 249, 86–102 (1983).
Wang, A. H.-J. & Rich, A. in Biological Macromolecules and Assemblies, Vol. 2 (eds McPherson, A. & Jurnak, F.) 128–170 (Wiley, New York, 1985).
Shakked, Z. & Kennard, O. in Biological Macromolecules and Assemblies, Vol. 2 (eds McPherson, A. & Jurnak, F.) 1–36 (Wiley, New York, 1985).
Shakked, Z. & Rabinovich, D. Prog. Biophys. molec. Biol. 47, 159–195 (1986).
Dickerson, R. E. & Drew, H. R. J. molec. Biol. 149, 761–786 (1981).
Fratini, A. V., Kopka, M. L., Drew, H. R. & Dickerson, R. E. J. biol. Chem. 257, 14686–14707 (1982).
Nelson, H. C. M., Finch, J. T., Luisi, B. F. & Klug, A. Nature 330, 221–226 (1987).
Drew, H. R. & Travers, A. A. Nucleic Acids Res. 13, 4445–4467 (1985).
Ulanovsky, L., Bodner, M., Trifinov, E. N. & Choder, M. Proc. natn. Acad. Set. U.S.A. 83, 862–866 (1986).
Diekmann, S. in Nucleic Acids and Molecular Biology Vol. 1 (eds Eckstein, F. & Lilley, D. M.) 138–156 (Springer, Berlin, 1987).
Hogan, M. E. & Austin, R. H. Nature 329, 263–266 (1987).
Hochschild, A. & Ptashne, M. Cell 44, 681–687 (1986).
Drew, H. R. & Travers, A. A. J. molec. Biol. 186, 773–790 (1985).
Elgin, S. C. R. Cell 27, 413–415 (1981).
Price, P. A., Moore, S. & Stein, W. H. J. biol. Chem. 244, 924–928 (1969).
Moore, S. in The Enzymes Vol. 14 (ed. Boyer, P. D.) 281–296 (Academic, New York, 1981).
Price, P. A. J. biol. Chem. 250, 1981–1986 (1975).
Sepersu, E. H., Shortle, D. & Mildvan, A. S. Biochemistry 26, 1289–1300 (1987).
Melgar, E. & Goldthwait, D. A. J. biol. Chem. 243, 4401–4408 (1968).
Melgar, E. & Goldthwait, D. A. J. biol. Chem. 243, 4409–4416 (1968).
Campbell, V. W. & Jackson, D. A. J. biol. Chem. 255, 3726–3735 (1980).
Crowther, R. A. in The Molecular Replacement Method (ed. Rossmann, M. G.) 174–178 (Gordon & Breach, New York, 1972).
Sussmann, J. L., Holbrook, S. R., Church, G. M. & Kim, S. H. Acta Crystallogr. A33, 800–804 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suck, D., Lahm, A. & Oefner, C. Structure refined to 2Å of a nicked DNA octanucleotide complex with DNase I. Nature 332, 464–468 (1988). https://doi.org/10.1038/332464a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/332464a0
This article is cited by
-
A cookbook for DNase Hi-C
Epigenetics & Chromatin (2021)
-
Divalent-metal-dependent nucleolytic activity of Cu, Zn superoxide dismutase
JBIC Journal of Biological Inorganic Chemistry (2006)
-
Assembly and function of a bacterial genotoxin
Nature (2004)
-
Deoxyribonuclease-Inhibitory antibodies in systemic lupus erythematosus
Journal of Biomedical Science (2003)
-
Structure and function of nucleases in DNA repair: shape, grip and blade of the DNA scissors
Oncogene (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.